
CasNo: 153-18-4
MF: C27H30O16
Appearance: pale-yellow crystalline solid
|
History |
In the mid-1930s, Hungarian scientist Szent Gyorgy firstly separated the flavonoid mixture. After the German pharmacy firstly made it into ranosine in 1942, the concept of vitamin P has been established worldwide. Further study proved that rutin was the most important flavonoids of vitamin P. These compounds were certified to have effects on many diseases in medical.Recently, the research of rutin mainly focuses on the extraction process improvement, pharmacological effects, and pharmacodynamics research, aiming at improvement of its bioavailability through the development of different dosage forms. As for the extraction process, new extraction and purification methods have been developed since the original alkali extraction acid precipitation method. These methods greatly improve its extraction efficiency and reduce cost, including hot water precipitation, hot water extraction with macroporous resin adsorbing purification, ultrasonic radiation, hot water extraction with alcohol precipitation, cold alkali percolation extraction with acid precipitation, continuous reflux extraction, ethanol extraction, supercritical CO2 extraction, and enzymatic hydrolysis .In recent years, advanced rutin dosage forms, such as rutin cyclodextrin saturation, HPMC controlled release tablets, solid dispersion tablets, coprecipitate, and rutin effervescent particles, greatly improve the rutin dissolution rate and its bioavailability. |
|
Pharmacology |
As a flavonoid substance, rutin has a significant protective effect on the cardiovascular system, including the endothelium-dependent vasodilation through NO-guanylate cyclase pathway, antagonization on platelet-activating factor (PAF), inhibition on subsequent reactions induced by PAF binding to its specific membrane receptor, and protection of myocardial cells .Rutin also has good free radical scavenging effects. Studies showed that rutin and its derivatives had a strong free radical scavenging effect, of which rutin possessed the strongest antioxidant activity. Rutin removed superoxide anion and hydroxyl radicals, exerted a strong anti-lipid peroxidation, protected mitochondria, and enhanced the activity of superoxide dismutase (SOD). |
|
Purification Methods |
The vitamin crystallises from MeOH or water/EtOH, dry it in air, then dry it further for several hours at 110o or in high vacuum at 120o. It forms yellow crystals from EtOH/Me2CO/H2O (2:1:1). It has also been purified by passing (0.5g) through a Kieselgel column (30 x 5cm) with EtOAc/MeOH/H2O (100:20:15), and after 750mL have passed through, the yellow fraction of 250mL gives the glycoside (0.3g) on evaporation. [H.rhammer et al. Chem Ber 101 1183 1968, Marini-Bettòlo Gazz Chim Ital 80 631 1950, Beilstein 18/5 V 519.] |
|
Chemical Structure and Properties |
Rutin is a rutinoside derived from quercetin, with glucose and rhamnose sugar groups attached to the hydroxy group at position C-3. It is classified as a flavonol glycoside and is found in various plants, including buckwheat, tobacco, forsythia, hydrangea, and viola. |
|
Therapeutic Properties |
Rutin is considered a natural flavonoid compound with various therapeutic potentials. It has antiplatelet, antiviral, and antihypertensive properties. |
|
Neuroprotective Effects |
Rutin has demonstrated neuroprotective effects in conditions such as brain ischemia, hypoxia, glutamate, and oxidative stress. It attenuates ischemic neural apoptosis, reduces lipid peroxidation, and enhances endogenous antioxidant defense enzymes. |
|
Anti-inflammatory Effects |
Rutin suppresses the activity of proinflammatory cytokines by reducing TNF-伪 and IL-1尾 production in microglia. |
|
Modulation of Hypercholesterolemia |
Rutin acts as a selective and non-toxic modulator of hypercholesterolemia. In studies conducted on animal models, rutin significantly reduced plasma triglyceride levels and total cholesterol levels. |
|
Sources |
Rutin was first discovered in buckwheat and is considered a major dietary source of this compound. It is widely distributed in vegetables and fruits, contributing to its consumption as part of a healthy diet. Buckwheat, in particular, is rich in rutin and has been found to prevent oxidative damage and exhibit antihypertensive effects. |
|
Physical properties |
Appearance: light yellow or yellow-green needle crystal or crystalline powder, tastes slightly bitter, usually contains three crystal water, melting point at 176– 178 °C. Solubility: Rutin is soluble in methanol, pyridine, alkaline solution, and boiling water and hardly soluble in cold water, chloroform, carbon disulfide, ether, benzene, and petroleum ether. |
|
Definition |
ChEBI: A rutinoside that is quercetin with the hydroxy group at position C-3 substituted with glucose and rhamnose sugar groups. |
InChI:InChI=1/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-33,35-38H,7H2,1H3/t8?,15?,17-,18+,20-,21+,22?,23?,26+,27-/m0/s1
Chemical investigation of the aerial par...
Disclosed is a method for improving the ...
The invention relates to a method for re...
Use of an MPO inhibitor for the treatmen...
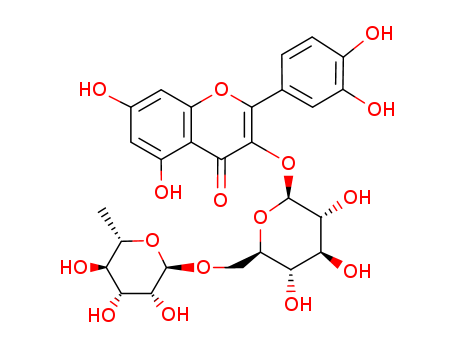
quercetin 3-O-<β-D-apiofuranosyl(1->2)α-L-rhamnopyranosyl(1->6)>-β-D-glucopyranoside)

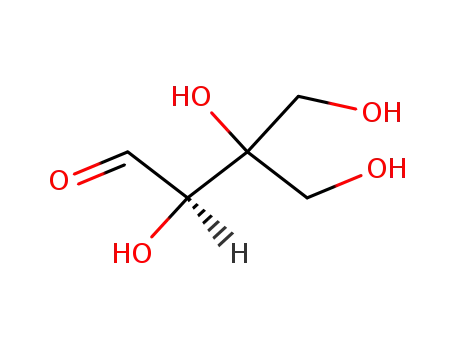
D-apiose

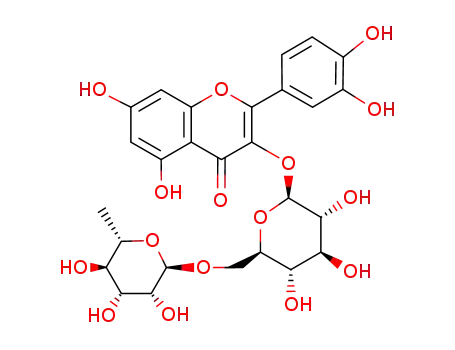
rutin
| Conditions | Yield |
|---|---|
|
With trifluoroacetic acid;
|
pasta seca

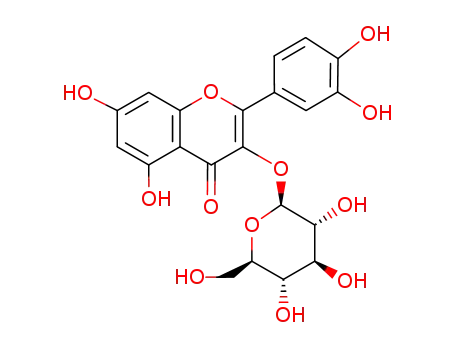
isoquercetin

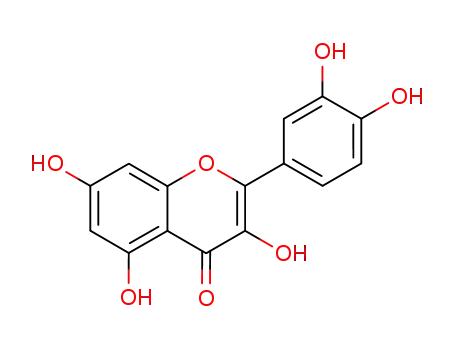
quercetol


rutin
| Conditions | Yield |
|---|---|
|
With ammonium bicarbonate; In acetic acid methyl ester; water; at 45 ℃; for 0.75h; Purification / work up;
|
|
|
In acetic acid methyl ester; water; at 45 ℃; for 0.5h; Purification / work up;
|
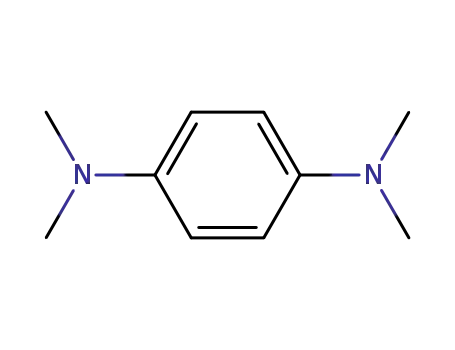
N,N,N',N'-tetramethyl-para-phenylenediamine
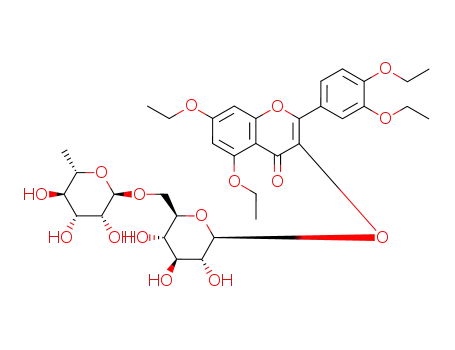
2-(3,4-Diethoxy-phenyl)-5,7-diethoxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxymethyl)-tetrahydro-pyran-2-yloxy]-chromen-4-one
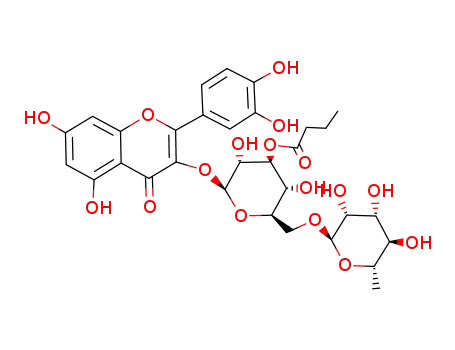
3''-O-butanoylrutin
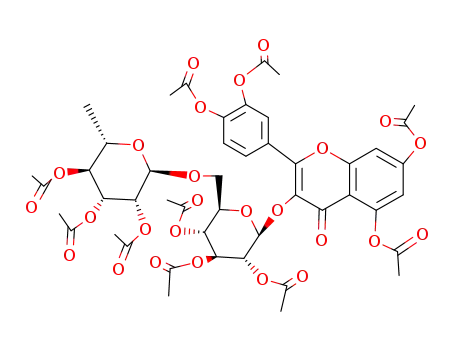
(2S,3R,4S,5R,6R)-2-((5,7-diacetoxy-2-(3,4-diacetoxyphenyl)-4-oxochroman-3-yl)oxy)-6-(((2R,3R,4R,5S,6S)-3,4,5-triacetoxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyltetrahydro-2H-pyran-3,4,5-triyl triacetate
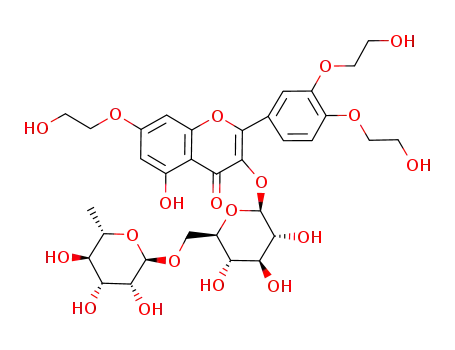
troxerutin