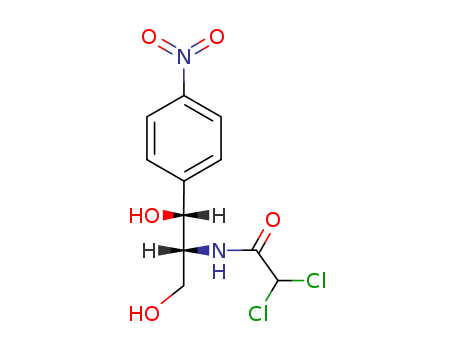

CasNo: 56-75-7
MF: C11H12Cl2N2O5
Appearance: White to grey-white crystalline powder
Buy Quality Chloramphenicol 56-75-7 In Stock with Immediately Delivery We supply high quality Chloramphenicol (CAS 56-75-7), in stock, factory directly supply to clients, lower prices, more competitiveness.
Chloramphenicol is White to grey-white crystalline powder, while it's Molecular Formula is C11H12Cl2N2O5. Chloramphenicol is unusual nitroaromatic metabolite produced by Streptomyces venezuelae, first published in 1947. Chloramphenicol is a broad spectrum antibiotic with good activity against Gram negative and anaerobic bacteria. Although restricted to ocular use, antibiotic resistance to other classes has refocused attention on this class. Chloramphenicol acts by binding to the 23S sub-unit of the 50S ribosome, inhibiting protein synthesis. Chloramphenicol has been extensively studied with over 35,000 literature citations.
The CAS number of Chloramphenicol is 56-75-7.
More information of Chloramphenicol 56-75-7 are:
|
Synonyms |
Acetamide, 2, 2-dichloro-N-[2-hydroxy-1- (hydroxymethyl)-2-(4-nitrophenyl)ethyl]- , [R-(R*,R*)]-;Tega-Cetin;Tevcosin;D(-)-threo-2-Dichloroacetamido-1-p-nitrophenyl-1,3-propanediol;Chlorocaps;D-(-)-threo-1-p-Nitrophenyl-2-dichloracetamido-1,3-propanediol;Isophenicol;Comycetin;Acetamide, 2, 2-dichloro-N-[.beta.-hydroxy-.alpha.- (hydroxymethyl)-p-nitrophenet hyl]-, D-threo-(-)-;CPh;Synthomycine;Anacetin;Amphicol;Acetamide, 2, 2-dichloro-N-[.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenet hyl]-,D-(-)-threo-;Mycinol;Synthomycetin;D-threo-N-(1, 1-Dihydroxy-1-p-nitrophenylisopropyl)dichloroacetamide;Leukomyan;Medichol;Otophen;Ophtochlor;Biocetin;D-(-)-Chloramphenicol;Chlorocid;Chloro-25 vetag;D-(-)-threo-alpha, alpha-Dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)acetamide;Chlomin;2,2-dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide;Halomycetin;Enteromycetin;Ronphenil;Pantovernil;Myclocin;Cloramidina;Viceton;Levoplast;D-(-)-threo-2, 2-Dichloro-N-[.beta.-hydroxy-.alpha.-(hydroxymethyl)]-p-nitrophene thylacetamide;Cloramficin;Tifomycin;Austracol;Alficetyn;Chloricol;Paraxin;Dextromycetin;Chloramfilin;Soluthor;Cloromisan;Ismicetina;I 337A;Ciplamycetin;Chloroptic;Chloramphenicol Levo/palmitate/L-base;D-threo-Chloramphenicol;Chlorocol;Chlorocidin C;(-)-Chloramphenicol;Catilan;Chloramphenicol-levo;D-threo-N-Dichloroacetyl-1-p-nitrophenyl-2-amino-1,3-propanediol;Farmicetina;Biophenicol;Chloromycetin (TN);Chloroject L;Tiromycetin;Loromisin;Pentamycetin;Ambofen;Doctamicina;Cloramicol;2,2-Dichloro-N-(2-hydroxy-1-(hydroxymethyl)-2-(4-(hydroxy(oxido)amino)phenyl)ethyl)acetamide, (1R, 2R)-;;Chlomycol;Sintomicetina;GlovesGloveticol;Chlora-Tabs;Aquamycetin;2,2-dichloro-N-[(1S,2R)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide;Chlorocide;Leukomycin;Acetamide,2,2-dichloro-N-[(1R,2R)-2- hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)- ethyl]-;Oleomycetin;Klorocid S;Loromisan;Detreomycine;Hortfenicol;Chloramsaar;Novomycetin;Micochlorine;Normimycin V;Isicetin;Chlorovules;Prestwick_51;Sno-Phenicol;D-threo-1-(p-Nitrophenyl)-2-(dichloroacetylamino)-1,3-propanediol;Chlornitromycin;Rivomycin;Chloramficin;Clorosintex;Acetamide, 2, 2-dichloro-N-[.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenet hyl];Isopto fenicol;Desphen;Intramycetin;Levomitsetin;Tifomycine;Unimycetin;Microcetina;Mychel;Glorous;Detreomycin;Chloromax;Leukamycin;D-(-)-2, 2-Dichloro-N-(.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenyl -ethyl)acetamide;Chemicetin;Chemicetina;Chloromycetin;Novophenicol;Levomycetin; |
|
CAS Number |
56-75-7 |
|
Molecular Formula |
C11H12Cl2N2O5 |
|
Molecular Weight |
323.133 |
|
Density |
1.547 g/cm3 |
|
Melting Point |
148-150 °C(lit.) |
|
Boiling Point |
644.913 °C at 760 mmHg |
|
Flash Point |
343.831 °C |
|
HS CODE |
2941400000 |
|
PSA |
115.38000 |
|
LogP |
1.82310 |
|
Pka |
11.03±0.46(Predicted) |
Chloramphenicol was originally produced by fermentation of Streptomyces venezuelae, but its comparatively simple chemical structure soon resulted in several efficient total chemical syntheses. With two asymmetric centers, it is one of four diastereomers, only one of which (1R,2R) is significantly active. Because total synthesis produces a mixture of all four, the unwanted isomers must be removed before use. Chloramphenicol is a neutral substance that is only moderately soluble in water, because both nitrogen atoms are nonbasic under physiologic conditions (one is an amide and the other a nitro moiety). It was the first broad-spectrum oral antibiotic used in the United States and was once very popular. Severe potential blood dyscrasia has greatly decreased its use in North America. Although its cheapness and efficiency makes it still very popular in much of the rest of the world where it can often be purchased over-the-counter without a prescription
InChI:InChI=1/C11H12Cl2N2O5/c12-10(13)11(18)14-8(5-16)9(17)6-1-3-7(4-2-6)15(19)20/h1-4,8-10,16-17H,5H2,(H,14,18)/t8-,9?/m1/s1
Articles related to Chloramphenicol:
|
Article |
Source |
|
Catalytic Syn-Selective Nitroaldol Approach to Amphenicol Antibiotics: Evolution of a Unified Asymmetric Synthesis of (-)-Chloramphenicol, (-)-Azidamphenicol, (+)-Thiamphenicol, and (+)-Florfenicol |
Chen, Fener,Cheng, Dang,Huang, Huashan,Jiang, Meifen,Liu, Minjie,Qu, Hongmin,Xia, Yingqi,Xiong, Tong,Zhang, Yan , p. 11557 - 11570 (2021/09/02) |
|
Unified Strategy to Amphenicol Antibiotics: Asymmetric Synthesis of (-)-Chloramphenicol, (-)-Azidamphenicol, and (+)-Thiamphenicol and Its (+)-3-Floride |
Liu, Jinxin,Li, Yaling,Ke, Miaolin,Liu, Minjie,Zhan, Pingping,Xiao, You-Cai,Chen, Fener , p. 15360 - 15367 (2020/11/30) |
WLP INGREDIENT INC is a quality supplier and manufacturer of Chloramphenicol . You can buy high quality, low price Chloramphenicol 56-75-7 here. Contact us.