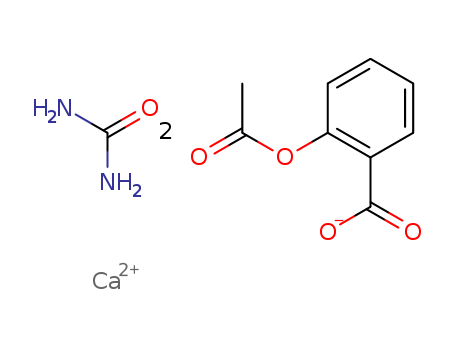

CasNo: 5749-67-7
MF: Ca(C9H7O4)2.CH4N2O
Appearance: White or almost white crystalline powder
|
Manufacturing Process |
500 g of finely powdered acetylsalicylic acid and 160 g of calcium carbonate (precipitated chalk), are intimately mixed and 3,000 cc of water are added. The mixture is stirred for 15 minutes or until the reaction is completed, which is indicated by the cessation of the liberation of carbon dioxide. The temperature is desirably maintained below 20°C by any suitable means. The mass is allowed to settle until the supernatant liquor is almost clear; this usually takes about 5 minutes, and the mixture is then filtered to remove unreacted material. This part of the process is carried out as quickly as possible so as to minimize any tendency of the calcium aspirin to hydrolyze in the solution. The filtrate is cooled to about 10°C and 1 to 1.5 volumes of 97% methanol, or pure wood alcohol is added. This causes the calcium aspirin to precipitate and the mass is then filtered to remove as thoroughly as possible the mother liquor. The residue of calcium aspirin is then suspended in a quantity of methanol equivalent to the volume previously used as a precipitant, and it is allowed to stand there for one hour or more with occasional or continuous agitation. The mass is again filtered, the filtrate being employed for the precipitation of calcium aspirin in a later batch. After the filtering of the first wash liquor, the calcium aspirin is again suspended in another quantity of methanol of an equivalent volume. This constitutes the second wash and it is carried out in the same way as the first wash. The filtrate is employed as a first wash in a later batch and this filtrate in turn is used, as is the filtrate of the first wash, for the precipitation of more calcium aspirin. Fresh alcohol is used as a new wash in a later batch and the washes are carried out in series. After the second wash the calcium aspirin is dried in a suitable manner, as by passing dry warm air over it, the temperature not being allowed to rise to such an extent as to decompose the aspirin; preferably the temperature is not permitted to rise above 50°C, but should be high enough to avoid deposition of water vapor, and the drying is completed when there is no longer an odor of methanol. |
InChI:InChI=1/2C9H8O4.CH4N2O.Ca/c2*1-6(10)13-8-5-3-2-4-7(8)9(11)12;2-1(3)4;/h2-5H,1H3,(H,11,12);2-5,11H,1H3;(H4,2,3,4);/q;-1;;+2/p-1
The invention belongs to the technical f...
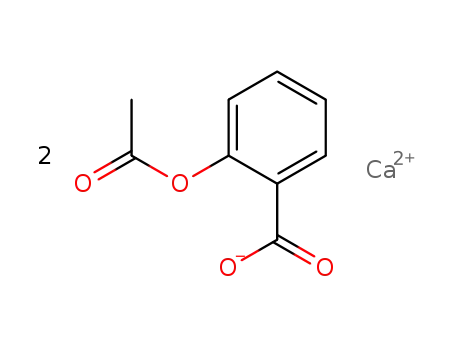
calcium O-acetylsalicylate

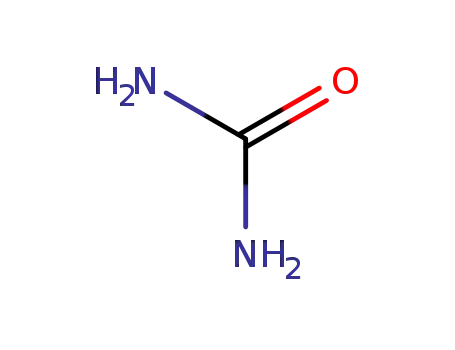
urea

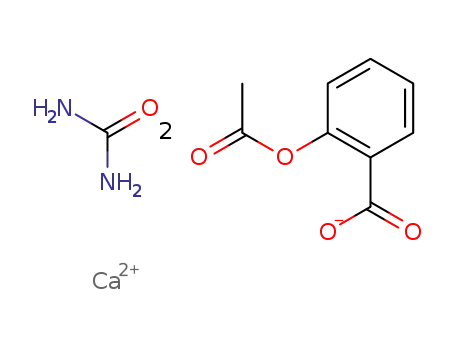
bis(2-acetoxybenzoic acid) calcium urea
| Conditions | Yield |
|---|---|
|
In water; for 0.0833333h; Industrial scale;
|
89.6 kg |

bis(2-acetoxybenzoic acid) calcium urea

salicylic acid
| Conditions | Yield |
|---|---|
|
bis(2-acetoxybenzoic acid) calcium urea; With sulfuric acid; In water; at 20 ℃; pH=1 - 2; Large scale;
With sodium carbonate; In water; at 90 - 95 ℃; for 0.666667h; pH=8; pH-value; Large scale;
|
175.1 kg |

calcium O-acetylsalicylate

urea

salicylic acid