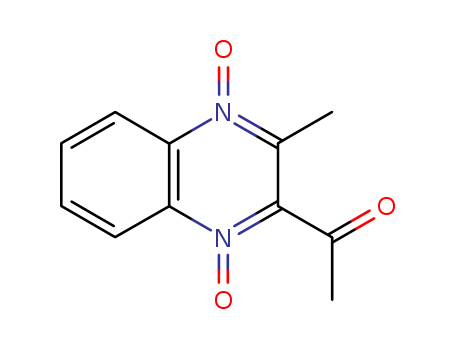

CasNo: 13297-17-1
MF: C11H10 N2 O3
InChI:InChI=1/C11H10N2O3/c1-7-11(8(2)14)13(16)10-6-4-3-5-9(10)12(7)15/h3-6H,1-2H3
A mild and highly efficient, environment...
The following standard molar enthalpies ...
A series of quinoxaline 1,4-di-N-oxide d...
-
The invention provides a preparation met...
Oxidation of o-nitroaniline with sodium ...
The invention relates to a preparation m...
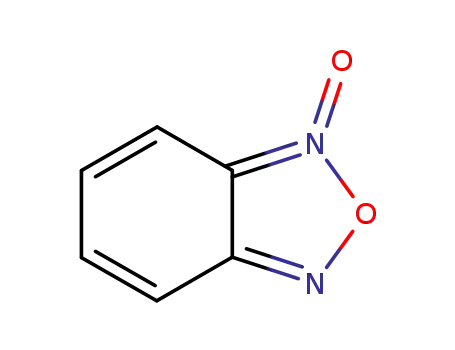
benzofurazan oxide

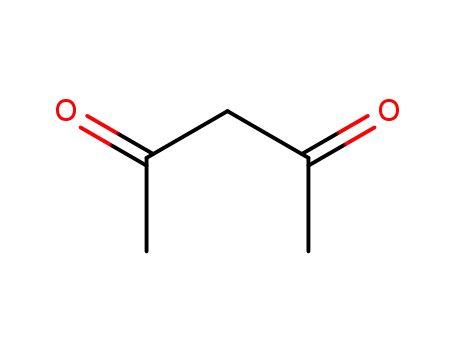
acetylacetone

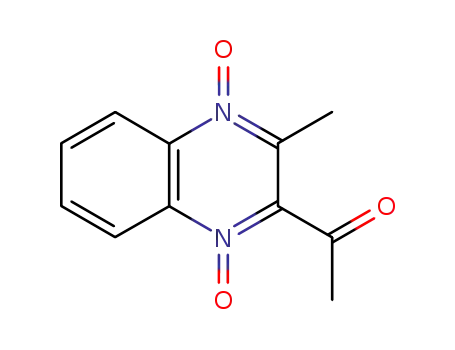
3-methyl-2-acetylquinoxaline-1,4-dioxide
| Conditions | Yield |
|---|---|
|
With triethylamine; In ethanol; at 45 ℃; for 2h;
|
94% |
|
With triethylamine; In ethanol; for 3h; Ambient temperature;
|
86% |
|
With silica gel; for 0.0666667h; Microwave irradiation;
|
85% |
|
With triethylamine; In dichloromethane; at 25 ℃; for 12h;
|
80% |
|
With triethylamine; at 60 ℃; for 4h; Temperature;
|
79.3% |
|
With triethylamine; In ethanol; at 20 ℃; for 24h;
|
78% |
|
With triethylamine; In chloroform; at 20 ℃; for 24h;
|
73% |
|
benzofurazan oxide; With β‐cyclodextrin; In methanol; water; for 0.5h;
acetylacetone; In methanol; water; at 20 ℃; for 0.333333h;
|
61% |
|
With Wako gel C-200; for 168h;
|
58% |
|
With 3 A molecular sieve; In ethanol; at 90 ℃;
|
58% |
|
With trimethylamine; for 24h; Ambient temperature;
|
|
|
In triethylamine;
|
|
|
With aminoethyl alcohol; calcium chloride;
|
|
|
at 20 - 35 ℃; for 20h;
|

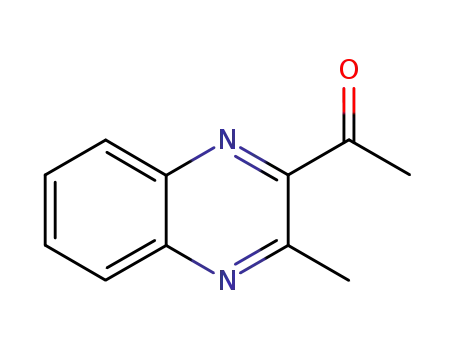
2-methyl-3-acetylquinoxaline


3-methyl-2-acetylquinoxaline-1,4-dioxide

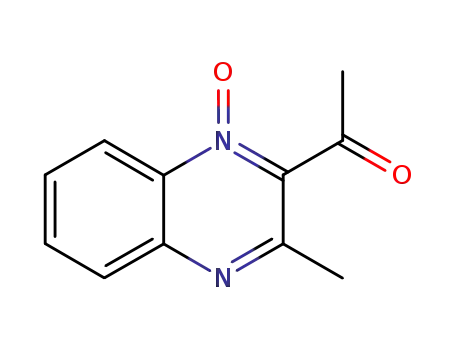
2-Acetyl-3-methylquinoxaline 1-Oxide
| Conditions | Yield |
|---|---|
|
With xylene; for 18h; Heating;
|
55.5% 2.3% 19.8% |

benzofurazan oxide

acetylacetone
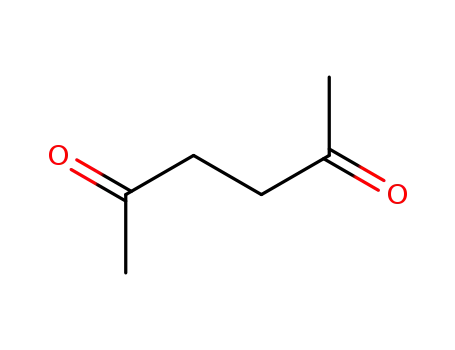
2,5-hexanedione
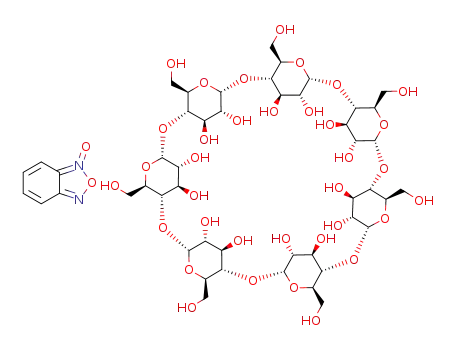
C6H4N2O2*C42H70O35
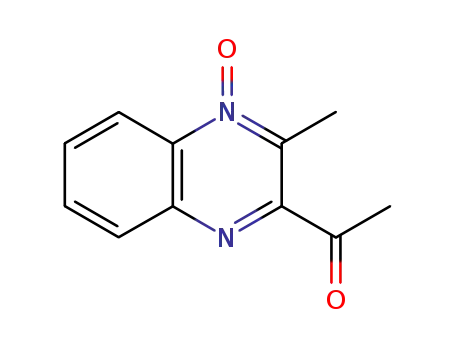
3-Acetyl-2-methylquinoxaline 1-Oxide

2-methyl-3-acetylquinoxaline

2-Acetyl-3-methylquinoxaline 1-Oxide
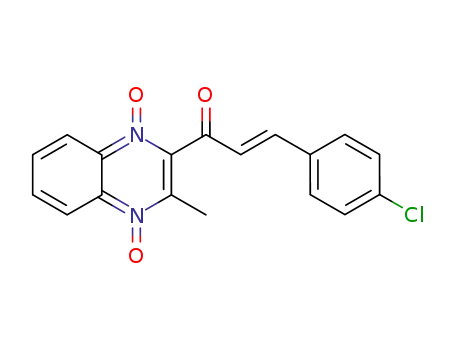
2-[3-(4-chlorophenyl)-2-propenoyl]-3-methylquinoxaline-1,4-dioxide