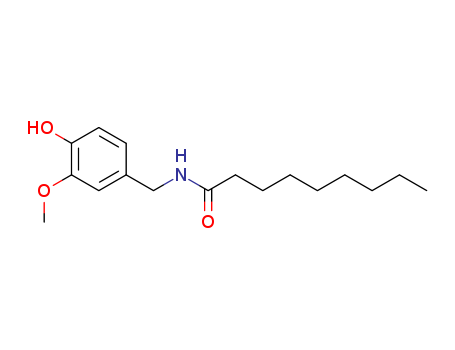

CasNo: 2444-46-4
MF: C17H27NO3
Appearance: solid
|
Preparation |
From nonanyl chloride and vanillylamine. |
|
references |
1. m. j. caterina, m. a. schumacher, m. tominaga, t. a. rosen, j. d. levine and d. julius, nature 1997, 389, 816-824. 2. y. g. gil and m. k. kang, life sci 2008, 82, 997-1003. 3. y. s. lee, d. h. nam and j. a. kim, cancer lett 2000, 161, 121-130. 4. h. c. chang, s. t. chen, s. y. chien, s. j. kuo, h. t. tsai and d. r. chen, hum exp toxicol 2011, 30, 1657-1665. 5. k. c. brown, t. r. witte, w. e. hardman, h. luo, y. c. chen, a. b. carpenter, j. k. lau and p. dasgupta, plos one 2010, 5, e10243.> |
|
Definition |
ChEBI: A capsaicinoid that is the carboxamide resulting from the formal condensation of the amino group of 4-hydroxy-3-methoxybenzylamine with the carboxy group of nonanoic acid. It is the active ingredient in many pepper sprays. |
InChI:InChI=1/C17H27NO3/c1-3-4-5-6-7-8-9-17(20)18-13-14-10-11-15(19)16(12-14)21-2/h10-12,19H,3-9,13H2,1-2H3,(H,18,20)
The invention relates to a capsaicin pre...
The invention provides a preparation met...
The invention belongs to the technical f...
The invention discloses a vanilla amide ...
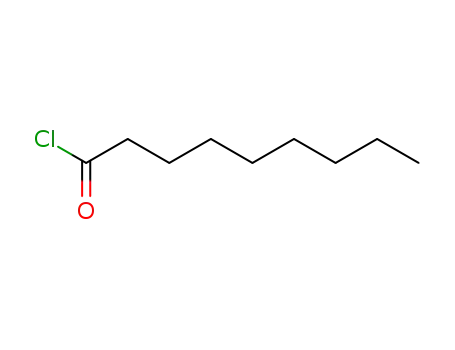
Nonanoyl chloride

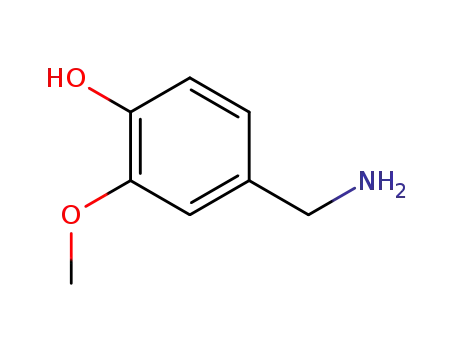
Vanillylamin

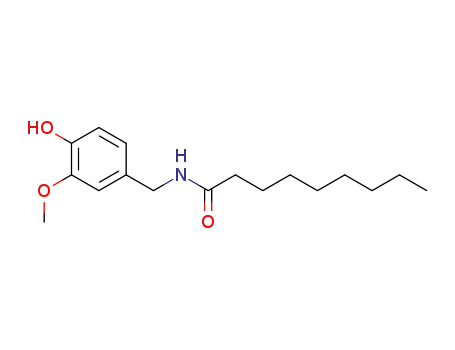
Nonivamide
| Conditions | Yield |
|---|---|
|
Vanillylamin; With sodium hydrogencarbonate; In chloroform; water; at 20 ℃; for 0.75h;
Nonanoyl chloride; In chloroform; water; at 20 - 40 ℃; for 1h;
|
94% |
|
With N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20 ℃;
|
88.6% |
|
In diethyl ether; N,N-dimethyl-formamide; Ambient temperature;
|
59% |
|
With diethyl ether;
|
|
|
With triethylamine;
|

Nonanoyl chloride

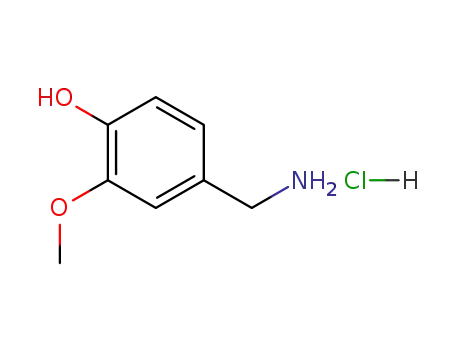
vanillylamine hydrochloride


Nonivamide
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In dichloromethane; water; at 20 - 42 ℃; for 2.5h; Temperature;
|
91.5% |
|
vanillylamine hydrochloride; With sodium hydroxide; In water; N,N-dimethyl-formamide; at 20 ℃; for 1h; Inert atmosphere; Cooling with ice;
Nonanoyl chloride; In tetrahydrofuran; water; N,N-dimethyl-formamide; at 20 ℃; for 18h; Inert atmosphere; Cooling with ice;
|
60.51% |

Nonanoyl chloride

Vanillylamin
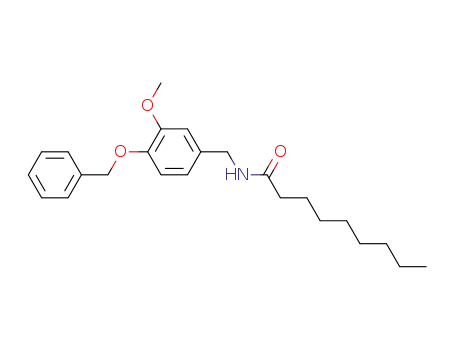
N-(4-benzyloxy-3-methoxy-benzyl)-nonanamide
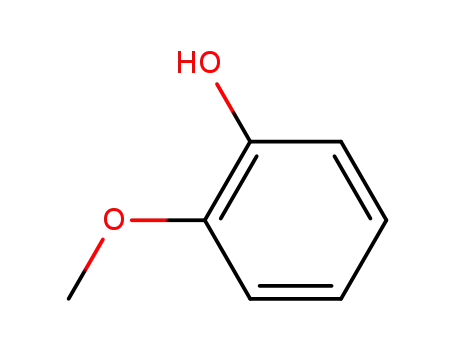
2-methoxy-phenol
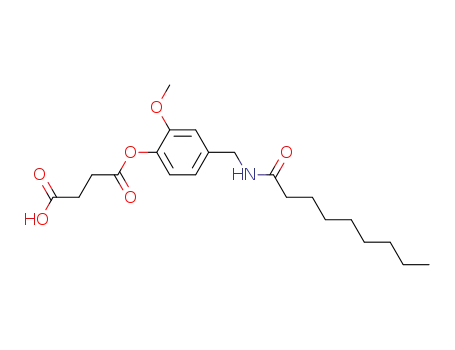
N-(4-O-succinic acid-3-methoxybenzyl)-nonamide
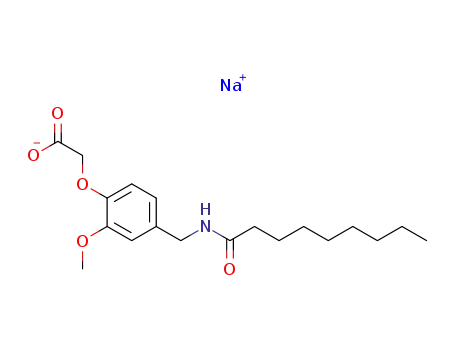
sodium nonivamide acetate
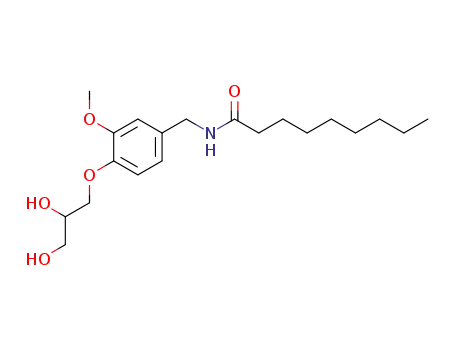
Glyceryl novinamide
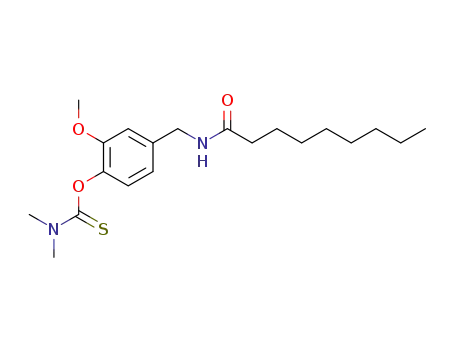
N-<4-<