
CasNo: 22373-78-0
MF: C36H61NaO11
Appearance: light cream amorphous powder
|
Biochem/physiol Actions |
Na+ ionophore; blocks glycoprotein secretion; may induce catecholamine secretion from chromaffin cells. Useful in potentiometric and spectroscopic studies of alkali metal ion complexes. |
|
Purification Methods |
Crystallise it from EtOH/H2O [Cox et al. J Am Chem Soc 107 4297 1985]. |
|
General Description |
Monensin is a polyether ionophoric antibiotic, which is produced by Streptomyces cinnamonensis. It is used to treat bacterial, fungal and parasitic infections. Monensin prevents the growth of colon cancer cells. It facilitates the transport of sodium and potassium ions between intracellular and extracellular spaces. Monensin prevents coccidiosis?in poultry production. |
InChI:InChI=1/C36H62O11.Na/c1-10-34(31-20(3)16-26(43-31)28-19(2)15-21(4)36(41,18-37)46-28)12-11-27(44-34)33(8)13-14-35(47-33)17-25(38)22(5)30(45-35)23(6)29(42-9)24(7)32(39)40;/h19-31,37-38,41H,10-18H2,1-9H3,(H,39,40);/q;+1/p-1/t19-,20+,21+,22+,23+,24+,25-,26-,27+,28-,29-,30-,31-,33+,34-,35+,36-;/m0./s1
Gibbs functions, enthalpies, entropies, ...
Measurements are reported on the stabili...
-
The incorporation of sodium - and (S)--p...
The incorporations of varicus carbon-13 ...
C36H61O11(1-)

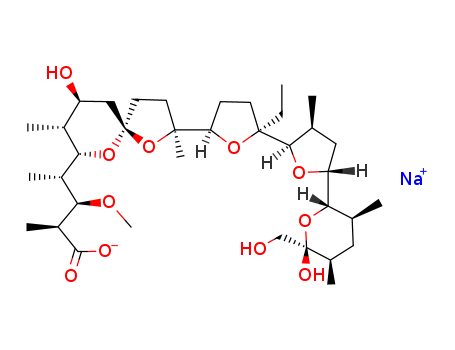
monensin A sodium salt
| Conditions | Yield |
|---|---|
|
With sodium cation; at 25 ℃; Thermodynamic data; ΔH-, Γ-, TΔS-;
|
monensin(1-) ion

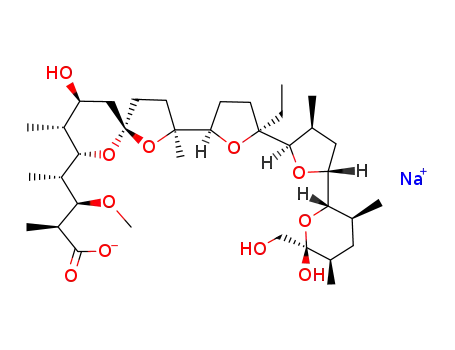
monensin sodium salt
| Conditions | Yield |
|---|---|
|
With sodium cation; In ethanol; at 25 ℃; Rate constant;
|

C36H61O11(1-)
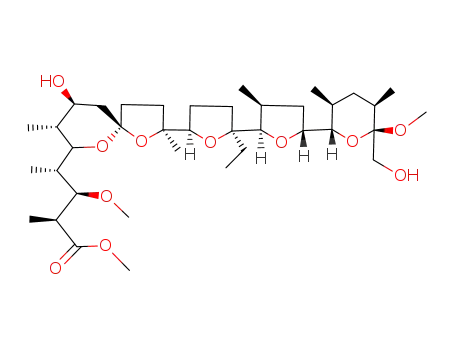
C38H66O11
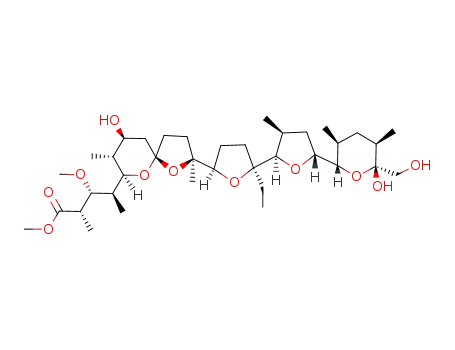
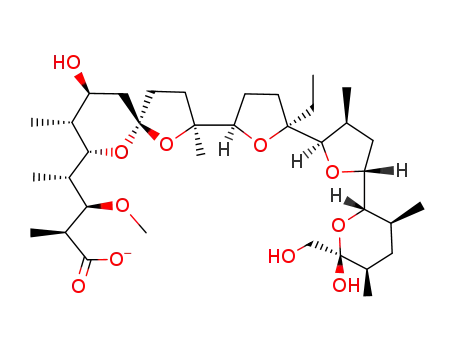
monensin methyl ester
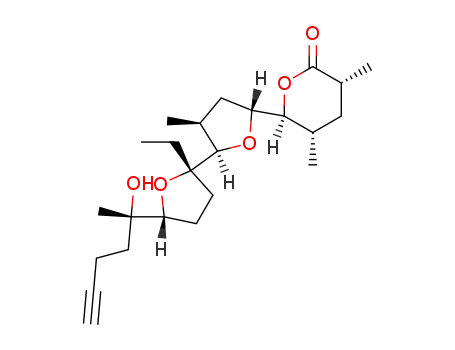
(3R,5S,6S)-6-[(2R,3S,5R,2'S,5'R)-2'-Ethyl-5'-((S)-1-hydroxy-1-methyl-pent-4-ynyl)-3-methyl-octahydro-[2,2']bifuranyl-5-yl]-3,5-dimethyl-tetrahydro-pyran-2-one
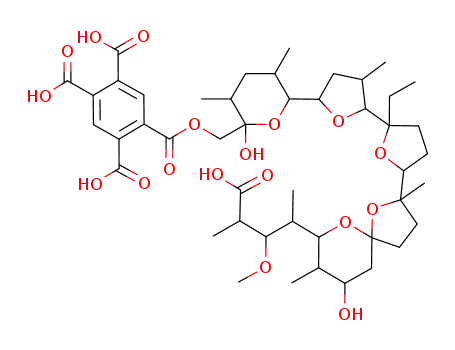
monensin 26-pyromellitate
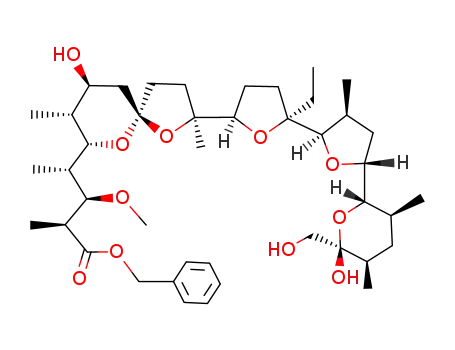
monensin A benzyl ester
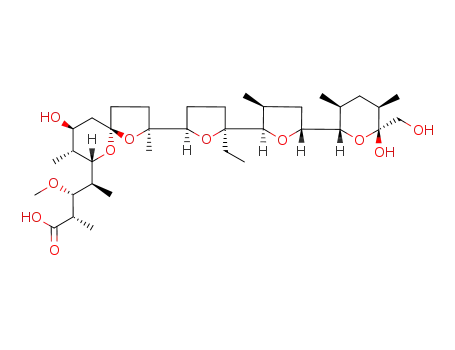
monensin
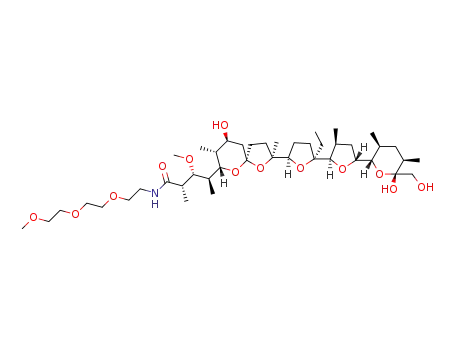
C43H77NO13