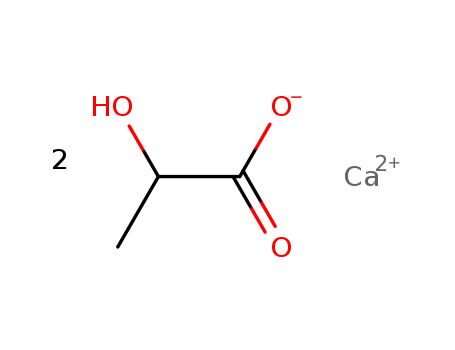

CasNo: 814-80-2
MF: C6H10CaO6
Appearance: White powder.
|
Production Methods |
Calcium lactate is prepared commercially by neutralization with calcium carbonate or calcium hydroxide of lactic acid obtained from fermentation of dextrose, molasses, starch, sugar, or whey. |
|
Hazard |
A poison. |
|
Pharmaceutical Applications |
Calcium lactate is used as a bioavailability enhancer and nutrient supplement in pharmaceutical formulations. A spray-dried grade of calcium lactate pentahydrate has been used as a tablet diluent in direct compression systems, and has been shown to have good compactability. The properties of the pentahydrate form have been considered superior to those of calcium lactate trihydrate when used in direct compression tablet formulations. Tablet properties may be affected by the hydration state of the calcium lactate and particle size of the material: reducing particle size increased crushing strength, whereas storage of tablets at elevated temperature resulted in dehydration accompanied by a reduction in crushing strength. Calcium lactate has also been used as the source of calcium ions in the preparation of calcium alginate microspheres for controlled- release delivery of active agents. It has been shown to result in lower calcium concentrations in the finished microspheres when compared with calcium acetate. Therapeutically, calcium lactate has been used in preparations for the treatment of calcium deficiency. |
|
Safety |
Calcium lactate was found to have no toxic or carcinogenic effects when dosed at levels of 0%, 2.5%, and 5% in drinking water to male and female rats for 2 years. |
|
storage |
Calcium lactate can exist in a number of hydration states, which are characterized as anhydrous, monohydrate, trihydrate, and pentahydrate. Dehydration of the pentahydrate form is rapid at temperatures of 558℃ and above. Dehydration is reported to be accompanied by some loss of crystallinity. Tablet crushing strength was reported to be reduced following dehydration of calcium lactate pentahydrate. |
|
Purification Methods |
Crystallise it from warm water (10mL/g) by cooling to 0o. [Beilstein 3 IV 636.] |
|
Incompatibilities |
Calcium salts, including the lactate, can display physical incompatibility with phosphate in the diet or therapeutic preparations, for example in enteral feed mixtures. |
|
Regulatory Status |
GRAS listed except for infant foods/formulas. Accepted as a food additive in Europe. Calcium lactate (anhydrous) is included in the FDA Inactive Ingredients Database (vaginal, tablet). It is used in oral dosage forms. Included in vaginal pessary formulations licensed in the UK. |
|
Who Evaluation |
Evaluation year: 1974 |
InChI:InChI=1/2C3H6O3.Ca/c2*1-2(4)3(5)6;/h2*2,4H,1H3,(H,5,6);/q;;+2/p-2
A calcium phosphate powder has been synt...
Mono- and bimetallic iridium complexes i...
An objective of the present invention is...
The present invention provides a polylac...
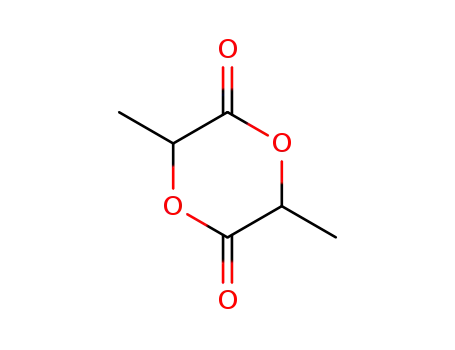
D,L-lactide

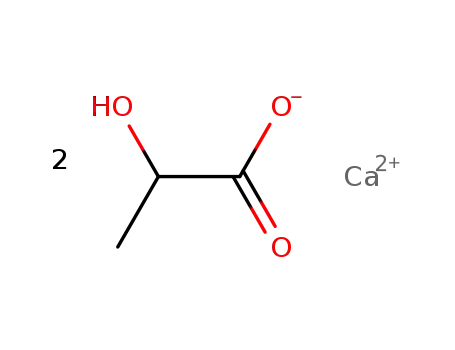
calcium lactate
| Conditions | Yield |
|---|---|
|
D,L-lactide; In water; at 130 - 170 ℃; for 2.83333h; Sealed tube;
With calcium carbonate; In water; for 0.166667h; Reagent/catalyst; Temperature; Heating;
|
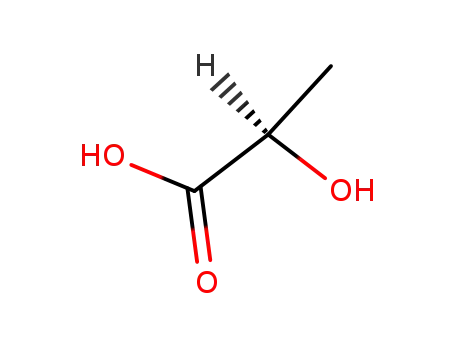
D-Lactic acid


calcium lactate
| Conditions | Yield |
|---|---|
|
With calcium hydroxide; In water;
|
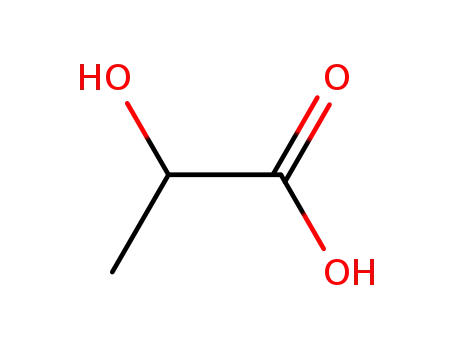
LACTIC ACID

D-Lactic acid

hydrogen cyanide
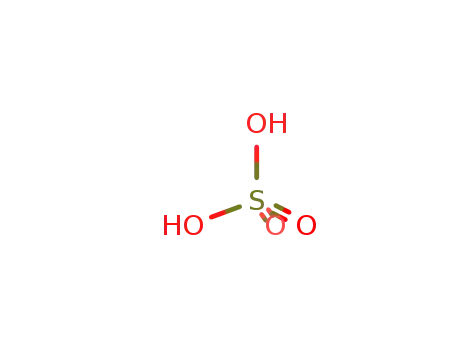
sulfuric acid
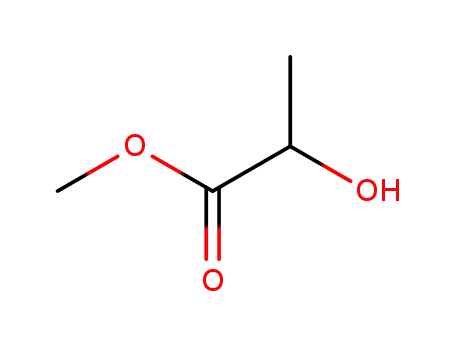
methyl lactate
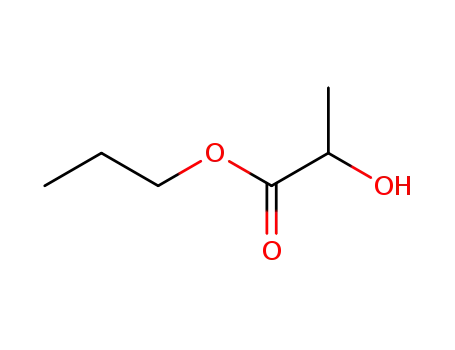
n-propyl lactate
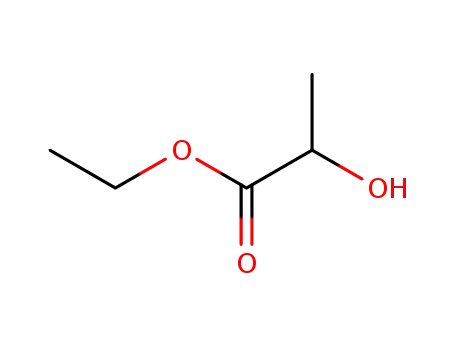
ethyl 2-hydroxypropionate
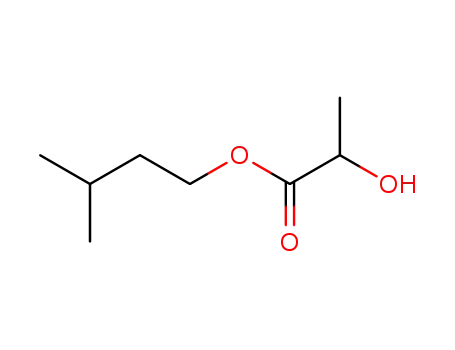
isopentyl lactate