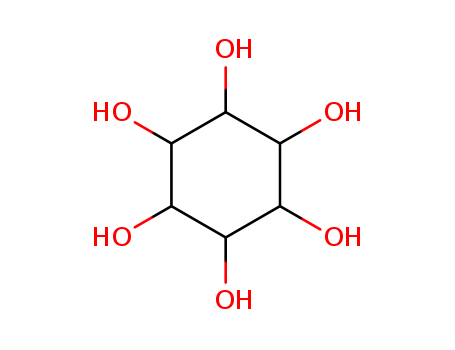
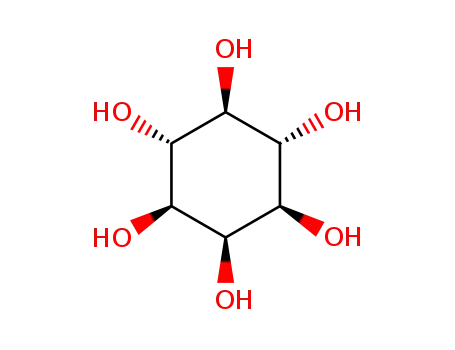
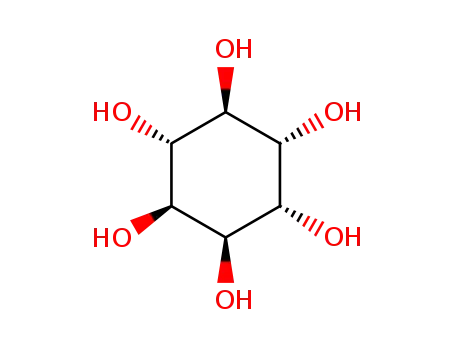
99% Purity Commercial production D-chiro-inositol 643-12-9 with Cheapest Price
- Molecular Formula:C6H12O6
- Molecular Weight:180.158
- Appearance/Colour:off-white powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:230 °C
- Refractive Index:1.784
- Boiling Point:291.326 °C at 760 mmHg
- PKA:12.63±0.70(Predicted)
- Flash Point:143.387 °C
- PSA:121.38000
- Density:2.038 g/cm3
- LogP:-3.83460
D-(+)-CHIRO-INOSITOL(Cas 643-12-9) Usage
|
Biochem/physiol Actions
|
Important second messenger in insulin signal transduction.
|
InChI:InChI=1/C6H12O6/c7-1-2(8)4(10)6(12)5(11)3(1)9/h1-12H/t1-,2-,3-,4-,5+,6+/m0/s1
643-12-9 Relevant articles
Transformation of cyclohexene to enantiopure cyclitols mediated by sequential oxyselenenylation with (S,S)-hydrobenzoin: Synthesis of D-chiro-inositol and muco-quercitol
Kim, Kwan Soo,Park, Jong H.,Moon, Hoi Kyung,Yi, Hann
, p. 1945 - 1946 (2007/10/03)
Oxyselenenylation of cyclohexene with (S...
Microbial Oxidation of Aromatics in Enantiocontrolled Synthesis. Part 1.Expedient and General Asymmetric Synthesis of Inositols and Carbohydrates via and Unusual Oxidation of a Polarized Diene with Potassium Permanganate
Hudlicky, Tomas,Mandel, Martin,Rouden, Jacques,Lee, Robert S.,Bachmann, Bryan,et al.
, p. 1553 - 1568 (2007/10/02)
This paper reports on the details of a g...
Optically active phenoxypropionic esters
-
, (2008/06/13)
Optically active compounds of the formul...
General Synthesis of Inositols by Hydrolysis of Conduritol Epoxides Obtained Biocatalytically from Halogenobenzenes: (+)-D-chiro-Inositol, allo-Inositol, muco-Inositol and neo-Inositol
Mandel, Martin,Hudlicky, Thomas
, p. 741 - 744 (2007/10/02)
Four of the nine isomeric inositols have...
643-12-9 Process route
-
- 488-54-0,488-55-1,488-58-4,488-59-5,551-72-4,576-63-6,643-10-7,643-12-9,6917-35-7,18685-70-6,38876-99-2,39907-99-8,40461-73-2,41546-32-1,41546-33-2,41546-34-3,41546-35-4,41546-36-5,87-89-8
D-chiro-inositol
Conditions
| Conditions |
Yield |
|
With water; platinum; Hydrogenation;
|
|
-
- 6090-97-7
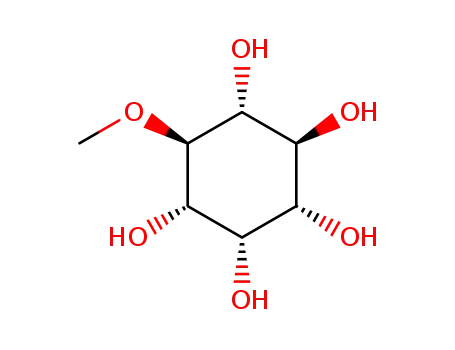
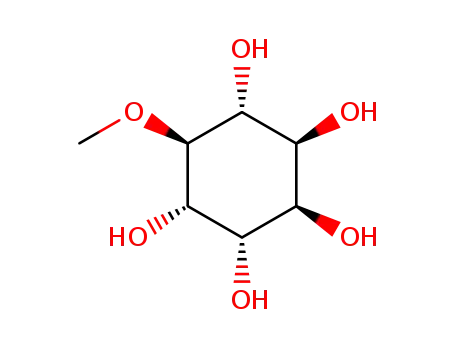
(1R,2S,3R,4S,5S,6S)-6-methoxycyclohexane-1,2,3,4,5-pentol
-
- 488-54-0,488-55-1,488-58-4,488-59-5,551-72-4,576-63-6,643-10-7,643-12-9,6917-35-7,18685-70-6,38876-99-2,39907-99-8,40461-73-2,41546-32-1,41546-33-2,41546-34-3,41546-35-4,41546-36-5,87-89-8
D-chiro-inositol
Conditions
| Conditions |
Yield |
|
In water; for 48h; Product distribution; biosynthesis with Simmondsia chinensis, various time;
|
|
643-12-9 Upstream products
-
38876-99-2
1L-chiro-inositol
-
488-54-0
D-chiro-inositol
-
488-59-5
myo-inositol
-
1254-38-2
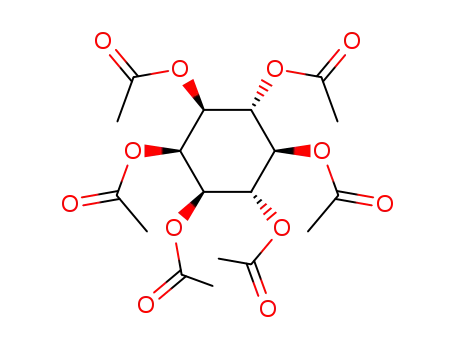
inositol peracetate
643-12-9 Downstream products
-
1254-38-2
inositol peracetate
-
866941-90-4
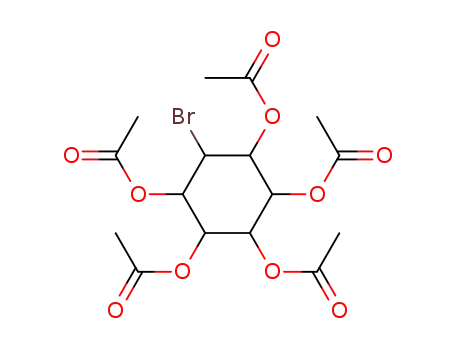
1,2,3,4,5-pentaacetoxy-6-bromo-cyclohexane
-
849585-22-4
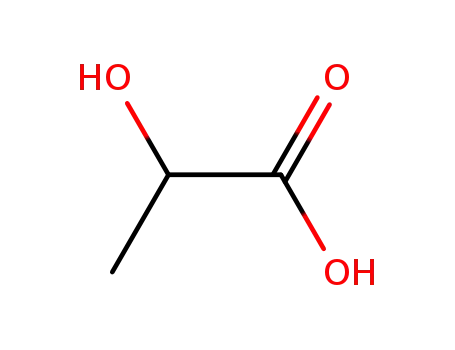
LACTIC ACID
-
319-89-1
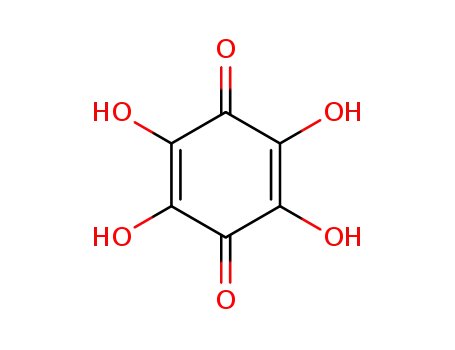
tetrahydroxy-1,4-quinone