
CasNo: 518-82-1
MF: C15H10O5
Appearance: red-orange powder
|
Physical and chemical properties |
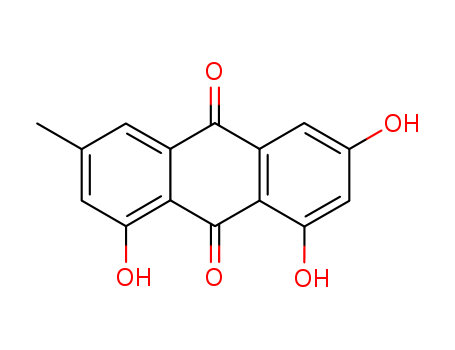
Emodin, also known by the Chemical name 1, 3, 8-trihydroxy-6-methyl-anthraquinone with a molecular formula of C15H10O5 and a molecular weight of 270.24, exists in the form of free emodin or emodin glycosides in rhizomes and roots of Rheum palmatum L, Rheum officinale Baill, and Rheum tanguticum Maxim. ex Balf. in the family Polygonaceae. In a form of orange-yellow crystals of melting point of 256-257℃ that can sublimate under a vacuum of 1,600 Pa, the compound is soluble in ethanol, sodium hydroxide, sodium carbonate and aqueous ammonia, slightly soluble in ether, chloroform, carbon tetrachloride and benzene, and almost insoluble in water. Its form as emodin triacetate is yellow crystals of melting point of 197℃, and the form of emodin 3-methyl ether (also known as physcion) is dark-red needle crystals of melting point of 207 ℃. The compound can be obtained by chemical synthesis or by extraction from plants. Aloe-emodin is one of the main components of aloe essential oil, present in the form of aloe-emodin in aloe, or present in the form of glycosides in rhubarb, senna and aloe. |
|
Radix et Rhizoma Rhei |
Radix et Rhizoma Rhei is the dry root or rhizome of Rheum palmatum L, Rheum officinale Baill, and Rheum tanguticum Maxim. ex Balf. in the genus Rheum of the family Polygonaceae. Rheum tanguticum Maxim. ex Balf has slender pinnate leaves with three deep lobes and dense inflorescence branches, often erect, clinging to the stem, which are the main difference of it from Rheum palmatum L. The main difference between Rheum tanguticum Maxim. ex Balf. and the other two species mentioned above are its lobed leaves with big serrate or broadly triangular margin, large and yellow-white flowers, oval-shaped flower buds and patulous branches. There are about 60 Rheum species all over the world, of which about 50 are in China. Morphological Characteristics: herbaceous perennial about two meters high. Rhizomes and roots are fleshy and yellowish-brown in color. The stem is erect, smooth, hairless and hollow. The basal leaf has a fleshy, long and sturdy petiole, about as long as the leaf blade. The leaf blade is broadly ovate or nearly round in shape, up to 40 cm in diameter, palmate-cleft with three to five (or to seven) lobes and each lobe sometimes also palmate-cleft or serrate. The leaf base is slightly heart-shaped. The stem leaf is small, shortly stalked. The ocrea is membranous and densely pubescent. Habitat and distribution: Grow in mountain areas, forest margins or grassland, wild or cultivated and distributed in Shaanxi, southeastern Gansu, Qinghai, western Sichuan, northwestern Yunnan and Eastern Tibet. Cultivation: suitable for growing in places with cool and moist climate and with deep soil layer that contains humus-rich sandy loam or calcareous loam, rather than growing in cold places with high temperature and humidity. Harvest: harvest during September to October, select plants that have grown more than three years, dig up the rhizomes and roots, remove the leaves, stems, rootlets, scrape the bark and buds, and then dried in air or in an oven, or sliced and dried. Figure 1 is an image of the Radix et Rhizoma Rhei |
|
Chemical constituents |
There have been more than 130 compounds isolated and characterized from a variety species of Rheum, including anthraquinones and anthraquinone glycosides, anthrones, bianthrones and bianthrone glycosides, stilbenes and stilbene glycosides, gallate, naphthalene derivatives, chromanones and chromanone glycosides, banzylethylketone, tannins and so on. Among them the anthraquinones are the most important and representative ingredients. Free anthraquinones mainly include rhein, emodin, physcion, aloe-emodin, and chrysophanol. And anthraquinone glycosides mainly include chrysophanol-1-glucoside, chrysophanol-8-glucoside, emodin-1-glucoside, emodin-8-glucoside, physcion-8-glucoside, physcion-8-gentiobiosiden, aloe-emodin-8-glucoside, aloe-emodin-3-glucoside, and rhein-8-glucoside. Bianthrone glycosides include sennosides A, B, C, D, E, and F. Stilbenes and stilbene glycosides include rhaponticin, rhapontigenin and de-oxyrhaponticin. Radix et Rhizoma Rhei contains a variety of tannins, both hydrolyzable tannins and condensed tannins[4]. Hydrolyzable tannins and its related compounds contain a variety of pentagalloylglucose and 1-O-galloyl-6-O-cinnamoyl-β-D-glucose. Condensed tannins and its related compounds contain catechin, epicatechin and its polymers. In addition, Radix et Rhizoma Rhei also contains a variety of banzylethylketone glycosides. |
|
Production method |
Emodin is a plant laxative widely present in plant organs such as roots of Radix et Rhizoma Rhei, bark and root bark of buckthorn and cassia seeds. Emodin can be extracted from roots and rhizomes of Radix et Rhizoma Rhei. It also can be obtained by synthesis, for example, using 2-methyl-anthraquinone, or 3, 5-nitro-phthalic anhydride and m-cresol as the raw material. |
|
Antitumor effect |
Pharmacological studies have found that anthraquinone derivatives in Radix et Rhizoma Rhei, rhein, emodin and aloe-emodin showed obvious inhibitory effect on cancers, especially on cancers such as melanoma, P388 leukemia and Ehrlich ascites carcinoma. The polysaccharides contained in Radix et Rhizoma Rhei could markedly inhibit the cells of sarcoma S180. And a concentration of 10 μg/ml emodin could reduce the maximum growth density and the mitotic index of human lung cancer cell line A-549, and apparently decreased the incorporation of tritiated thymidine and the level DNA content. It was also found that the drug could relatively increase the number of cells at the G1 and S stages and decreased the number of aneuploid cells as well as the number of cells at the G2/M phase, with a sinistral displacement of the peak in the DNA histogram. These results suggested that emodin has an obvious inhibitory effect on human lung cancer A-549 cells. The antitumor mechanism of Radix et Rhizoma Rhei is presently considered to be the inhibition of respiration and DNA biosynthesis of cancer cells. It is also believed that rhein and emodin may destroy the cancer cells directly. |
|
Diuretic effect |
Both rhein and emodin have evident diuretic effect. The urine volume increased to peak two to four hours after dosing, and in the meanwhile the amounts of Na+ and K+ discharges were also reached to the peak. Aloe-emodin and chrysophanol were less effective in diuretic property as compared with rhein and emodin. The mechanism is contributed to the inhibitory effect of rhein and emodin on renal medullary Na+, K+-ATPase. The Na+ re-absorption in renal tubules is mainly active transport which needs energy from ATP hydrolysis catalyzed by the Na+, K+-ATPase. When the enzyme is inhibited, Na+ re-absorption would decrease because of insufficient energy supply, which leads to water discharge increase along with the increase of Na+ discharge. When distal convoluted tubule Na+ increases, promote Na+-K+ exchange would be promoted, resulting in increase in K+ discharge. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Emodin may be sensitive to prolonged exposure to light. Probably a weak acid due to the phenolic functional groups. |
|
Health Hazard |
ACUTE/CHRONIC HAZARDS: When heated to decomposition Emodin emits acrid smoke and irritating fumes. |
|
Fire Hazard |
Flash point data for Emodin are not available; however, Emodin is probably combustible. |
|
Biological Activity |
Naturally occurring anthraquinone that displays a range of biological activities. Exhibits anti-inflammatory, antitumor and neuroprotective effects. |
|
Biochem/physiol Actions |
Cell permeable: yes |
|
Purification Methods |
Archin forms orange needles from EtOH, Et2O, *C6H6, toluene or pyridine. It sublimes above 200o at 12mm. [Tutin & Clewer J Chem Soc 99 946 1911, IR: Bloom et al. J Chem Soc 178 1959, UV: Birkinshaw Biochem J 59 495 1955, Raistrick Biochem J 34 159 1940.] 1R,2S-(-)Ephedrine see (-)-ephedrine (1R,2S-2-methylamino-1-phenylpropanol) in “Miscellaneous” in Chapter 6. |
|
Chemical properties |
Orange needles of melting point of 256-257℃ (259-260℃); soluble in alcohol, slightly soluble in ether, chloroform and benzene, and insoluble in water. The solution color would become cherry red when it is dissolved in aqueous caustic solutions, aqueous solution of sodium carbonate or ammonia solution. |
|
General Description |
Orange needles or powder. |
InChI:InChI=1/C15H10O5/c1-6-2-8-12(10(17)3-6)15(20)13-9(14(8)19)4-7(16)5-11(13)18/h2-5,16-18H,1H3
From the heartwood of Cassia sophera two...
Emodin, a precursor of chrysophanol in t...
Psorospermum febrifugum; Guttiferae; ger...
From branches of the shrub Plotarium att...
Emodin is a widely distributed anthraqui...
Members of the aldo-keto reductase and s...
Skyrin and rugulosin A are bioactive bis...
A catalyst-free method for the synthesis...
euxanmodin A

1,6,8-trihydroxy-3-methyl-9,10-anthraquinone

euxanthone
| Conditions | Yield |
|---|---|
|
With alkaline dithionite;
|
emodin 1-O-β-D-glucopyranosyl-(1→2)-glucopyranoside

D-glucose

1,6,8-trihydroxy-3-methyl-9,10-anthraquinone
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; water; In methanol; at 60 ℃; for 6h;
|
physcion
1,3,8-trimethoxy-6-methyl-9,10-anthraquinone
endocrocin
2-(2-hydroxy-4-methyl-benzoyl)-3,5-dimethoxy-benzoic acid
physcion
1,6,8-triacetoxy-3-methyl-9,10-anthraquinone
emodin-6-O-β-D-glucopyranoside
Chrysophanol