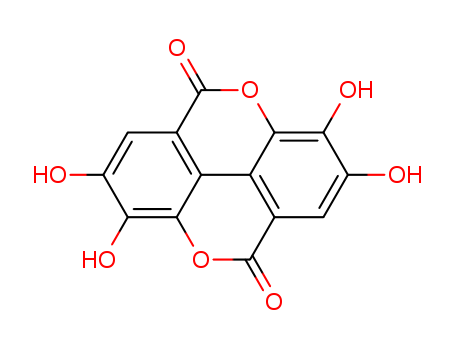

CasNo: 476-66-4
MF: C14H6O8
Appearance: cream to light yellow crystalline solid
|
|
|
|
Application |
Ellagic acid is used in medicine and cosmetics, as an antioxidant, and has anti-cancer and anti-viral effects.Ellagic acid, a common plant polyphenol, is an inhibitor of glutathione S-transferase with Exhibits antitumor activity. It can be used for the determination of factor XIIa in plasma. Contact activation can occur in blood coagulation. It is also used as selective, ATP-competitive inhibitor of casein kinase 2 and a Topo I and II, FGR, GSK, and PKA inhibitor. |
|
Definition |
ChEBI: Ellagic acid is an organic heterotetracyclic compound resulting from the formal dimerisation of gallic acid by oxidative aromatic coupling with intramolecular lactonisation of both carboxylic acid groups of the resulting biaryl. It is found in many fruits and vegetables, including raspberries, strawberries, cranberries, and pomegranates. It has a role as an antioxidant, a food additive, a plant metabolite, an EC 5.99.1.2 (DNA topoisomerase) inhibitor, an EC 5.99.1.3 [DNA topoisomerase (ATP-hydrolysing)] inhibitor, an EC 1.14.18.1 (tyrosinase) inhibitor, an EC 2.3.1.5 (arylamine N-acetyltransferase) inhibitor, an EC 2.4.1.1 (glycogen phosphorylase) inhibitor, an EC 2.5.1.18 (glutathione transferase) inhibitor, an EC 2.7.1.127 (inositol-trisphosphate 3-kinase) inhibitor, an EC 2.7.1.151 (inositol-polyphosphate multikinase) inhibitor, an EC 2.7.4.6 (nucleoside-diphosphate kinase) inhibitor, a skin lightening agent, a fungal metabolite, an EC 2.7.7.7 (DNA-directed DNA polymerase) inhibitor and a geroprotector. It is an organic heterotetracyclic compound, a cyclic ketone, a lactone, a member of catechols and a polyphenol. It is functionally related to a gallic acid. |
|
Preparation |
Ellagic acid is mainly extracted from plants, and it is present in several fruits such as cranberries, strawberries, raspberries, and pomegranates. Usually, the raw materials are degreasing and then extracted with an alkaline aqueous solution or extracted with ethanol. Then, after removing water-soluble proteins and gliadins, the sugar ligands can be decomposed by enzymes to obtain purer ellagic acid. |
|
General Description |
Cream-colored needles (from pyridine) or yellow powder. Odorless. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Ellagic acid reacts as a weak acid. Incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated. Heat is also generated by the acid-base reaction between phenols and bases. May be sulfonated exothermically very readily (for example, by concentrated sulfuric acid at room temperature). May be nitrated very rapidly, even by dilute nitric acid. |
|
Fire Hazard |
Flash point data for Ellagic acid are not available; however, Ellagic acid is probably combustible. |
|
Biological Activity |
Selective, ATP-competitive inhibitor of casein kinase 2 (CK2) (IC 50 values are 40, 2900, 3500, 4300 and 9400 nM for CK2, Lyn, PKA, Syk? and FGR respectively). Exhibits antioxidant, antitumor and anticarcinogenic activity and also inhibits glutathione S-transferase. |
|
Biochem/physiol Actions |
Commonly occurring plant polyphenol, inhibitor of glutathione S-transferase. Used for the assay of factor XIIa in plasma. Contact activation in blood coagulation. |
|
Anticancer Research |
Ellagic acid is a naturally occurring phenolic constituent present in natural productsand nuts, most elevated amounts of which are found in raspberries (Daniel et al.1990). EA is considered as a potent anticarcinogenic and antimutagenic compound.EA shows anti-angiogenic property by repressing PDGF-R movement and phosphorylationof its substrate. It can intrude with endothelial cell-associated VEGR-2phosphorylation bringing about the restraint of the downstream signaling activatedby this receptor and in the hindrance of two key events fundamental in angiogenesis,i.e., EC movement and morphogenic separation into capillary-like structure. In parallel,EA indicated robust inhibitory activity against perivascular cells through itsrestraint of PDGF-R action and signaling prompting hindrance of VSMC relocation(Labrecque et al. 2005).It is a phenolic compound extracted from pomegranate. It is an antiproliferative andantioxidant compound (Murakami et al. 1996). It induces apoptosis in cancer cellsof the prostate and breast and prevents the process of metastasis in different cancers(Singh et al. 2016b). |
|
Purification Methods |
This antioxidant crystallises from pyridine. It forms a dark green solution in aqueous N NaOH. The tetraactetate dilactone crystallises from Ac2O, with m 340o. [Beilstein 19 H 261, 19 III/IV 3164, 19/7 V 108.] |
InChI:InChI=1/C14H6O8/c15-5-1-3-7-8-4(14(20)22-11(7)9(5)17)2-6(16)10(18)12(8)21-13(3)19/h1-2,15-18H
Introduction Hydrolysable tannins occur ...
The application of oxidative dimerizatio...
A sustainable, reagent-less and one-pot ...
-
The first dimeric ellagitannins, rhoipte...
Two new hydrolyzable tannins, isotercheb...
The aqueous extract of acetone powder, w...
Two new ellagic acid glycosides were iso...
The aim of this study was to characteriz...
Ellagic acid derivatives possess antimic...
Amariin is an ellagitannin with two dehy...
5-O-galloyl-3,4-(S)-hexahydroxydiphenoyl proto-quercitol

(+)-proto-quercitol

3,4,5-trihydroxybenzoic acid

ellagic acid
| Conditions | Yield |
|---|---|
|
With sulfuric acid; at 90 ℃; for 12h;
|
1,5-di-O-galloyl-3,4-(S)-hexahydroxydiphenoyl proto-quercitol

(+)-proto-quercitol

3,4,5-trihydroxybenzoic acid

ellagic acid
| Conditions | Yield |
|---|---|
|
With sulfuric acid; at 90 ℃; for 12h;
|
3,4,5-trihydroxybenzoic acid
methyl galloate
Ethyl gallate
N-galloglycine
tetrabenzylellagic acid
3,3',4,4'-tetra-O-bemzyl-5,5'-di-C-benzylellagic acid
1,1,6-tribenzyl-3,7,8-tris-benzyloxy-1H-chromeno[5,4,3-cde]chromene-2,5,10-trione
2,3,7,8-tetrakis-benzoyloxy-chromeno[5,4,3-cde]chromene-5,10-dione