
CasNo: 67-45-8
MF: C8H7N3O5
Appearance: solid
|
Antibacterials |
Furazolidone is a kind of nitrofuran-class antibacterial agents which has certain antibacterial effects on gram positive and negative bacteria including Salmonella, Shigella, Escherichia coli, Klebsiella pneumoniae Peter, Enterobacter spp., Staphylococcus aureus, Enterococcus faecalis, Streptococcus pyogenes, Vibrio cholerae, Campylobacter, Bacteroides spp. It also has antibacterial activities against Trichomonas, Giardia lamblia at certain concentration. Its mechanism of action is by interfering with the bacterial oxidoreductase and thereby blocking the normal metabolism of bacteria. It is mainly used for treatment of various intestinal infections, dysentery, diarrhea, enteritis, Escherichia coli septicemia, typhoid, cholera, infectious rhinitis, blackhead, trichomoniasis, coccidiosis and Cartesian WBC. It can also be combined together with other drugs such as antacids for treatment of Helicobacter pylori-induced inflammation. Furazolidone has side effects on infants, such as liver and kidney damage, allergic reactions, etc., Thereby, it is generally recommended to minimize the use or avoid using on infants. |
|
Aquaculture Fungicides |
Furazolidone has a low toxicity to fish, turtles etc. It can be used for both prevention as well as treatment of early stage disease in the field of freshwater fish farming. Upon the water temperature being lower than 20 °C, rinse 20 to 30 minutes; at a temperature being higher than 20 °C, rinse 10 to 15 minutes. At the water temperature being close to 20 °C, Furazolidone, at a concentration of 0.025 ppm, can inhibit a variety of pathogens. For comparison, in the pool, tank-bred goldfish, or tropical fish can tolerate a concentration of 1.5ppm without die. Therefore, in case of serious illness, you can even use a concentration in the range of 0.4~1.0ppm; After sprinkling over the entire pool with the drugs, domestic fish should be more fed high-quality commercialized feed; ornamental fish must be fed with live animal foodstuffs such as live Daphnia, Cyclops, chironomid larvae, and tubificidae; improve the nutrients conditions of diseased fish and enhance their capability of disease resistance. For prevention of various kinds of disease of domestic fish such as bald white mouth disease, gill disease, stigmatosis, leprnorthsis, red skin disease, immerse and rinse one time before stocking; For prevention of diseases of ornamental fish such as bald white mouth disease, gill rot, leprnorthsis, and destroyed fin and tail, etc. For fish fed in a small body of water, immerse once a day, and rinse once again at the interval of one day. Treatment of various diseases of ornamental fish such as bald white mouth disease, gill rot, leprnorthsis, and destroyed fin and tail, immerse and rinse once daily, and rinse once again at the interval of one day; it can be continuously rinsed 3 to 5 times until the disappearance of symptoms and the recovery of the fish. Furazolidone residue will cause potential harm to humans, causing hemolytic anemia, multiple neuritis, eye damage and acute hepatic necrosis. Thereby it has been disabled in Chinese, EU and some other countries. The above information is edited by the lookchem of Dai Xiongfeng. |
|
Production methods |
Using ethanolamine as raw material, it undergoes condensation reaction with urea to obtain β-hydroxyethyl urea which is further converted into 3-nitroso-2-oxazolidinone through nitrification, and cyclization reaction. Further reduce it with iron powder, and then condense together with 5-nitro-2-furfural glycol acetate and formaldehyde to obtain the final product, furazolidone. |
|
Manufacturing Process |
In 212 cc of water are mixed 21.2 grams (0,112 mol) of N-(benzylidene)-3- amino-2-oxazolidone, 8.93 grams of concentrated sulfuric acid, and 30.1 grams (0.124 mol) of 5-nitro-2-furaldehyde diacetate. This mixture is heated to effect the hydrolysis of N-(benzylidene)-3-amino-2-oxazolidone, steam distillation of the benzaldehyde and hydrolysis of 5-nitro-2-furaldehyde diacetate. Approximately 1? hours are required for this reaction to take place. When the bulk of the benzaldehyde has been removed, 50 cc of 99% isopropanol are added, the reaction mixture is refluxed a short time, and the crystals of N(5-nitro-2-furfurylidene)-3-amino-2-oxazolidone are filtered from the hot suspension. The product is washed with water and isopropanol and dried; a yield of 23.3 grams, 92.8% based on N-(benzylidene)-3-amino-2- oxazolidone of MP 254° to 256°C is obtained, according to US Patent 2,759,931. |
|
World Health Organization (WHO) |
Furazolidone, a nitrofuran derivative with antibacterial and antiprotozoal activity, was introduced in 1954. In the 1970s it was shown to have a carcinogenic potential following long-term administration to experimental animals. However, the relevance of this to short-term therapy in man has not been established. The risk-benefit assessment varies and furazolidone remains widely available in many countries for the treatment of diarrhoea and enteritis. |
|
Antimicrobial activity |
It is active against a wide range of enteric pathogens, including Salmonella enterica, Shigella spp., enterotoxigenic Escherichia coli, Campylobacter jejuni, Aeromonas hydrophila, Plesiomonas shigelloides, Vibrio cholerae and V. parahaemolyticus. Yersinia enterocolitica is intrinsically resistant. Furazolidone is also active against the protozoa Giardia lamblia and Trichomonas vaginalis. |
|
Acquired resistance |
Acquired resistance has been observed in V. cholerae O1 and O139, S. enterica serotypes Typhi and Enteritidis, A. hydrophila and Shigella spp. Such resistance may be transferable, and there is cross-resistance with nitrofurantoin. Many of these reports come from the Indian subcontinent, where furazolidone is used widely for treating diarrheal diseases. |
|
Hazard |
A questionable carcinogen, use has been restricted. |
|
Pharmaceutical Applications |
A non-ionic synthetic compound, available for oral use only. It is poorly soluble in water (40 mg/L) and ethanol (90 mg/L), but dissolves well in dimethylformamide (10 g/L). It decomposes in the presence of alkali. |
|
Contact allergens |
Furazolidone belongs to the group of nitrofurans. This antimicrobial (antibacterial and antiprotozoal) agent is used in veterinary medicine both topically and orally, particularly in animal feed. Reactions are reported in workers exposed to it in animal feeds. Cross-reactions with other nitrofuran derivatives are rare. |
|
Biochem/physiol Actions |
Furazolidone induces interstrand cross-links in subsequent mutation in bacterial cells. It also inhibits mono and diamine oxidase activities in eukaryotes. |
|
Pharmacokinetics |
There is substantial absorption (65–70%) after oral administration, but the drug is heavily metabolized, so that only about 5% of the material excreted is microbiologically active. A dose of 5 mg/kg achieves a maximum plasma concentration of around 1 mg/L. Protein binding is about 30%. Intact drug can be found in various body fluids in concentrations approximating to the minimum inhibitory concentration (MIC) for various intestinal pathogens. Less than 1% of the drug is excreted into urine. |
|
Side effects |
Most reported side effects are mild and only rarely cause discontinuation of treatment. Nausea and vomiting are experienced by around 8% of patients. Other adverse events include neurological reactions (mainly headache; 1.3% of patients), ‘systemic’ reactions such as fever and malaise (0.6%) and skin rashes (0.54%). Administration of furazolidone may give rise to inhibition of monoamine oxidase, and disulfiram-like reactions have been reported. |
|
Safety Profile |
Poison by ingestion and intraperitoneal routes. Human systemic effects by ingestion: dyspnea, respiratory depression, and eosinophilta. Experimental reproductive effects. Human mutation data reported. Questionable carcinogen. When heated to decomposition it emits toxic fumes of NOx. |
|
Synthesis |
Furazolidone, 3-(5-nitrofurfuryliden)amino-2-oxazolidinone (33.7.8), is synthesized from 2-hydroazinoethanol, which is reacted with diethyloxalate to make 3- amino-2-oxazolidone. Reacting this with benzaldehyde gives the corresponding hydrazone (33.3.7). Purifying the resulting product and then reacting it with 5-nitrofurfurol gives furazolidone. |
|
Veterinary Drugs and Treatments |
Furazolidone is usually a drug of second choice in small animals to treat enteric infections caused by the organisms listed below. Because it is no longer commercially available (in the USA), it may be difficult to locate. |
|
Definition |
ChEBI: A member of the class of oxazolidines that is 1,3-oxazolidin-2-one in which the hydrogen attached to the nitrogen is replaced by an N-{[(5-nitro-2-furyl)methylene]amino} group. It has antibacterial and antiprotozoal properties, and is us d in the treatment of giardiasis and cholera. |
|
Brand name |
Benilen;B-fsudi;Carbopuradin;Dapecfuran;Dectolin;Dialidene;Diarexin;Diarin;Diclofur;Doreplston;Dushel;Enterar;Enteroxon;Framenterol;Ft 15;Furaberin;Furacol l.;Furalatin p.;Furalidan;Furaliqua;Furoxona-cp;Fuvitan;Fuxol;Fuzatyl;Galacid;Gamafur s.;Giarlin;Ginvel;Injecur;Intefuran;Kalpec-f;Lacolysat;Mastisept;Multi-med 2;Multi-med 3;Multi-med 6;Neforox alpha cpto;Neftivit;Nicolen r;Nifulin;Parkestress forte;Saleton;Scantrimon;Sibren;Sirben;Syralbuna;Tetrafur;Tranatogen-ova;Ufa-cfo-400;Uterojekt;Vagifurona;Vetoprim;Vsf-medical g 15. |
|
General Description |
Furazolidone is an effective antiprotozoal and antibacterial agent. |
InChI:InChI=1/C8H7N3O5/c12-8-10(3-4-15-8)9-5-6-1-2-7(16-6)11(13)14/h1-2,5H,3-4H2/b9-5+
A method of formulating a synthetic drug...
ESR spectra of anion radicals for 29 der...
New 3-Imino-1,2,4-benzotriazine-1-oxides...
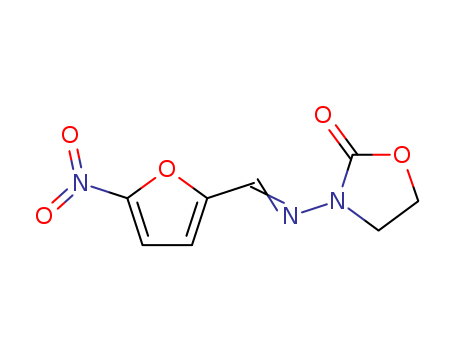
(2-imino-oxazolidin-3-yl)-(5-nitro-[2]furfuryliden)-amine

furazolidone
| Conditions | Yield |
|---|---|
|
With acetic acid; sodium nitrite;
|
|
|
With sulfuric acid; water;
|
5-nitro-furfural-[2-(2-hydroxy-ethyl)-semicarbazone]

furazolidone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: SOCl2; benzene
2: H2O; aqueous H2SO4
With thionyl chloride; sulfuric acid; water; benzene;
|
3-amino-2-oxazolidinone
5-nitrofurane-2-carboxaldehyde
phosgene
2-(2-(propan-2-ylidene)hydrazinyl)ethanol
N-(5-acetamido-2-furfurylidene)-3-amino-2-oxazolidone
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone
3-amino-2-oxazolidinone