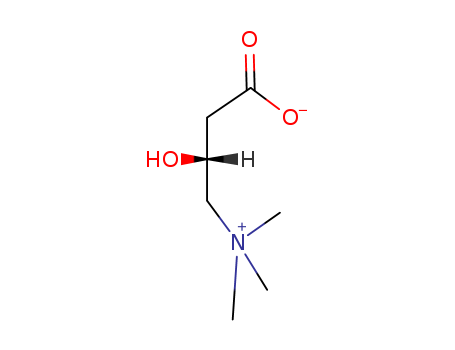

CasNo: 541-15-1
MF: C7H15NO3
Appearance: white crystalline powder
|
|
|
|
Role in Cellular Energy Metabolism |
Levocarnitine is an essential component in cellular energy metabolism, contributing to the transport of fatty acids into the mitochondria for energy production. |
|
Treatment for Valproic Acid Overdose |
Levocarnitine, the active isomer of carnitine, has been utilized in the treatment of valproic acid overdose, including cases resulting from both overdose and regular dosages of valproic acid. |
|
Bioavailability and Distribution |
While a single dose of levocarnitine does not persist in the system for an extended period, chronic intravenous administration in hemodialysis patients can lead to significant uptake into tissues, with muscle carnitine levels increasing by up to 200%. |
|
Effectiveness in Renal Anemia |
Studies have shown that levocarnitine treatment can ameliorate renal anemia and reduce the requirements for erythropoiesis-stimulating agents (ESAs) in hemodialysis patients. Multiple studies support the efficacy of levocarnitine in treating renal anemia. |
|
Beneficial Effects on Dyslipidemia |
Levocarnitine supplementation may benefit dialysis patients with dyslipidemia by enhancing the transport of free fatty acids into mitochondria and reducing their availability for triglyceride synthesis. |
|
Restoration of Carnitine Levels |
Levocarnitine supplementation has been found to restore normal plasma carnitine levels and alleviate symptomatic deficiencies with good tolerability. In hemodialysis patients, it increases plasma carnitine concentrations and improves fatigue as assessed by patients. |
|
Indications and Usage |
Carnitine is a type of vitamin B, and its structure is similar to that of amino acids. It is mainly used to help transport long-chain fatty acids to provide energy and to prevent fat from collecting in the heart, liver, and skeletal muscles. Carnitine can prevent disordered fat metabolism due to diabetes, fatty liver disease and heart disease, and it can reduce heart damage, lower blood triglyceride, aid in weight loss, and increase the antioxidant effects of vitamin E and C. Meats and giblets are high in carnitine. Artificially synthesized carnitine includes L-carnitine, D-carnitine, and DL-carnitine, and only L-carnitine has physiological activities. On the other hand, D-carnitine and DL-carnitine competitively inhibit the activity of carnitine acetyltransferase (CAT) and carnitine palmitoyltransferase (PTC) to prevent cells’ fat metabolism, thus harming human nutrition. L-carnitine was first discovered in 1905 by Russian chemists Gulewitsch and Krimberg in infusion broth, and its chemical structure was determined in 1927 by Tomita and Senju. L-carnitine is a white crystalline or transparent powder, and its melting point is 200℃ (decompose). It is easily soluble in water, lye, methanol and ethanol, barely soluble in acetone and acetate, and insoluble in chloroform. It is hygroscopic. L-carnitine can be used as an animal nutrition enhancer, and it is mainly used to enhance protein-based food additives to promote fat absorption and utilization. L-carnitine also is a nutrition enhancer that is mainly used in soy-based infant foods, sports nutritional foods and weight loss foods to promote fat absorption and utilization. According to China’s regulations, the permitted amount in biscuits, drinks, and dairy beverages is 600-3000mg/kg; in solid beverages, liquids, and gel capsules, 250-600mg/kg; in formula, 300-400mg/kg; in infant foods, 70-90mg/kg (1g tartrate is equivalent to 0.68g l-carnitine). L-carnitine can also be used as an appetite booster. L-carnitine affects the elimination and utilization of ketone bodies, so it can be used as a biological antioxidant to eliminate free radicals, maintain membrane stability, increase animal immunity and resistance to disease and stress. Oral L-carnitine can increase the speed of sperm maturation and sperm vitality, it can increase the number of forward-moving sperm and motile sperm in oligospermia and asthenospermia patients, thus increasing the women’s clinical pregnancy rate, and it does so safely and effectively. L-carnitine can bind with organic acids and the large amounts of acyl coenzyme derivatives produced in children with fatty acid metabolism disorder and turn them into water soluble acylcarnitine to be excreted through urine. This not only aids in controlling acute acidosis occurrences, but also effectively improves long-term prognosis. |
|
Mechanisms of Action |
L-carnitine cannot participate in protein biosynthesis, but it promotes ketone body utilization and nitrogen generation to an extent. Its main function is to promote fatty acid beta oxidation, which occurs in the liver and the mitochondria of other tissue cells. It is known that free fatty acids and acyl coenzyme A cannot penetrate the inner mitochondrial membrane, but acylcarnitine can do so swiftly. Thus, it is determined that L-carnitine is the carrier that transports fatty acid and acyl forms into the mitochondrial membrane. The mechanisms of this transporting process are still unknown, but it is certain that carnitine acyl-CoA transferase is the key enzyme in this process. It has two isoenzymes, one of which is carnitine acyl-CoA transferase I, positioned on the outer side of the membrane. When fatty acid is catalyzed by acyl-CoA-synthatase to produce acyl-CoA, it is transported by carnitine acyl-CoA transferase I into the membrane. After it has entered the membrane, it is catalyzed by the second isoenzyme - carnitine acyl-CoA transferase II – to turned into a form of acyl-CoA that can be directly utilized by fatty acid catabolic enzymes. Afterwards, it releases energy through processes such as dehydrogenation and deoxygenation. L-carnitine can also adjust the acyl ratio in mitochondria, thus affecting energy metabolism. L-carnitine can participate in the transportation of branched chain amino acid metabolites, which encourages the regular metabolism of branched chain amino acid. |
|
Pharmacokinetics |
L-Carnitine is very easily soluble in water, and can be entirely absorbed by the human body when consumed through food. It is known that the small intestine absorbs L-carnitine, but there is little known about the specific absorption process of carnitine (free or esterified) through intestine mucosa and about the specific absorption area. Besides external food sources of carnitine, humans can also synthesize carnitine with their own bodies. The liver and kidneys are mainly responsible for synthesizing carnitine. They progress from lysine into epsilon beta hydroxy three methyl lysine, and use aldolase and aldehyde oxidase to transform it into L-carnitine. Besides lysine, the body’s biosynthesis of L-carnitine also requires methionine, vitamin C, nicotinic acid and vitamin B6. A rat dissection showed that carnitine is most concentrated in the adrenal gland, followed by the heart, bones, muscles, fat tissue, and liver, and the carnitine concentration in the kidneys and brain are 40 times that in blood. Human carnitine concentration has varied greatly due to inconsistencies in measuring method and test subject. The biological method of testing human blood carnitine content placed it between 0.86-2.87mg/100ml, and the enzymology method of testing muscle carnitine content placed it between 0.457-2.479μg/g. The absorbed carnitine is metabolized by the human body and excreted in urine as free carnitine. |
|
Physical properties |
The appearance is white lens or white transparent fine powder, with a slight special fishy smell. Very soluble in water, ethanol and methanol, slightly soluble in acetone, insoluble in ether, benzene, chloroform and ethyl acetate. It is easy to absorb moisture, and will deliquesce or even liquefy when exposed to air. It can be placed in the solution with pH value of 3 ~ 6 for more than 1 year, and can withstand the high temperature of more than 200 ℃. Its combined bond and binding group have good water solubility and water absorption. The specific rotation is - 30 ± 1 °. |
|
Definition |
ChEBI: The (R)-enantiomer of carnitine. |
|
General Description |
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.Levocarnitine is a naturally available compound that plays a significant role in fatty acid oxidation and energy production in the human body. It is majorly found in skeletal and cardiac muscles of mammals and facilitates the transport of long chain fatty acids into mitochondria. |
|
Biochem/physiol Actions |
Carnitine is a quaternary amine that occurs naturally in most mammalian tissue. It is present in relatively high concentrations in skeletal muscle and heart where it is involved in regulating energy metabolism. It shifts glucose metabolism from glycolysis to glycogen storage and enhances the transport of long chain fatty acids into the mitochondria where they are oxidized for energy production. |
InChI:InChI=1/C7H15NO3/c1-8(2,3)5-6(9)4-7(10)11/h6,9H,4-5H2,1-3H3/t6-/m1/s1
The invention relates to the technical f...
The invention provides a micro-reaction ...
The first fluorescent probes that are ac...
The assignment of biochemical functions ...
4-(chloromethyl)oxetane-2-one

trimethylamine

(2E)-4-hydroxybut-2-enoic acid

L-carnitine

(S)-carnitin
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In water; at 0 - 20 ℃; for 2h; Product distribution / selectivity;
|
4-(chloromethyl)oxetane-2-one

trimethylamine

(2E)-4-hydroxybut-2-enoic acid

L-carnitine

(S)-carnitin
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In water; at 0 - 20 ℃; for 2h; Product distribution / selectivity;
|
CARNITINE
(2R)-(-)-3-cyano-2-hydroxypropyltrimethylammonium chloride
Toluene-4-sulfonate((R)-3-carboxy-2-hydroxy-propyl)-trimethyl-ammonium;
(R)-4-Bromo-3-hydroxy-butyric acid octyl ester
lauroyl carnitine chloride
(-)-O-Butyryl-carnitin
(-)-O-Caproyl-carnitin
o-palmitoyl-L-carnitine hydrochloride