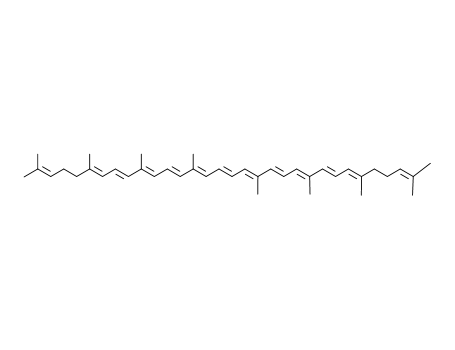

CasNo: 502-65-8
MF: C40H56
Appearance: Pink Powder
|
Production Methods |
Lycopene extract from tomato is produced from a tomato variety with high lycopene content, within the range of 150 to 250 mg/kg. This particular variety is not generally marketed for direct consumption, but is used primarily in the production of this lycopene extract. The extract is produced by crushing tomatoes into crude tomato juice that is then separated into serum and pulp. The tomato pulp is then extracted with ethyl acetate. The final product is obtained after solvent removal by evaporation under vacuum at 40-60°C. |
|
Biological Activity |
Lycopene may act as an inhibitor of tumor cells. In one study, lycopene was shown to inhibit PDGF-BB-induced signalling and cell migration in human cultured skin fibroblasts (Wu et al., 2007). Trapping of PDGF by lycopene compromised melanoma-induced fibroblast migration and attenuated signalling transduction in fibroblasts (Wu et al., 2007). In functional studies, lycopene inhibited melanoma-induced fibroblast migration in a noncontact coculture system and attenuated signalling in fibroblasts simulated by melanoma-derived conditioned medium (Chiang et al., 2007). |
|
Biochem/physiol Actions |
Antioxidant micronutrient of tomatoes associated with decreased risk for cancer and cardiovascular disease. Enhances gap juction communication between cells via upregulation of connexin 43 and reduces proliferation of cancer cells in culture. Inhibits cholesterol synthesis and enhances low-density lipoprotein degradation. |
|
Mechanism of action |
Lycopene is a red carotenoid compound found in pink grapefruit, papaya, wolfberry, goji, and tomatoes Dietary supplementation with tomato-based products appears to lower biomarkers of oxidative stress and carcinogenesis. Limited available evidence from small human intervention studies indicate that lycopene supplementation for 10–12 weeks may decrease UV-induced erythema. Although the bioavailability of lycopene in raw tomatoes is low due to tight binding with indigestible fiber, lycopene can be released from the food matrix through heating and food processing. The effect of topical lycopene is not well characterized. An in vivo study using SKH-1 hairless mice found that topical lycopene reduced the activity of ornithine decarboxylase (ODC) and myeloperoxidase (MPO), enzymes that have been implicated in the carcinogenic and acute inflammatory effect of UVB irradiation. |
|
Anticancer Research |
Lycopene is a naturally occurring chemical that manifests as a red pigment contained in common foods such as tomatoes, pink grapefruits, guava, and watermelon (Giovannucci 1999). This is a very strong antioxidant that has been found to prevent and even reverse the progression of prostate cancer, as well as treating benign prostatic hyperplasia. In a recent study, 30 mg a day of lycopene showed curative results in prostate cancer. For best results, supplements are recommended alongside eating and drinking plenty of lycopene-containing food and juices (Jatoi et al. 2007). Earlier research showed that taking a specific combination of lycopene, selenium, and saw palmetto by mouth for 8 weeks reduced pain in men with prostate swelling and pelvic pain more significantly than saw palmetto alone (Feifer et al. 2002).Lycopene shows anticancer activity against prostate, endometrial, breast, and colon carcinomas. It inhibits human cancer cell proliferation by activation of cancer-preventive enzymes like phase II detoxification enzymes, by suppression of insulin-like growth factor-I-stimulated growth (Wang et al. 2012). It also activates antioxidant enzymes like GST, GSH, and GPx and protects from oxidative stress caused by carcinogens. It alters PI3K/AKT pathway and ERK and Bcl-2 signaling in pancreatic and gastric carcinoma cells, respectively (Singh et al. 2016b). |
|
Purification Methods |
Crystallise lycopene from CS2/MeOH, diethyl ether/pet ether, or acetone/pet ether. Also purify it by column chromatography on deactivated alumina, CaCO3, calcium hydroxide or magnesia. It is oxygen sensitive and is stored in the dark, in an inert atmosphere. Also purified like -Carotene. [Beilstein 1 III 1076, 1 IV 1165.] |
|
Chemical Composition and Sources |
Lycopene is a tetraterpene compound belonging to the carotenoid family. Abundantly found in tomatoes and tomato-based products. Also present in other fruits and vegetables, responsible for their yellow, orange, and red pigmentation. |
|
Health-Promoting Properties |
While not considered an essential nutrient, lycopene is recommended for dietary intake due to its health-promoting properties. Acts as an antioxidant, protecting lipids, proteins, and DNA from oxidative damage. |
|
Stability and Isomerization |
Lycopene structure is sensitive to thermal treatment and oxidative processes. Vulnerable to light, oxygen, high temperature, acids, catalysts, and metal ions. |
|
Absorption and Factors Influencing Absorption |
Absorption is similar to dietary fat absorption. Influenced by age, gender, hormonal status, smoking, alcohol, and other dietary components. |
|
Role as an Antioxidant |
Prevents autoxidation of fats and related products, contributing to its importance as an antioxidant. |
|
Production and Uses |
Commercially available through chemical synthesis, fermentation, or isolation from natural sources like algae, fungi, and plants. Used in functional foods, cosmetics, and cosmeceutical products due to its biological properties. |
|
Definition |
ChEBI: An acyclic carotene commonly obtained from tomatoes and other red fruits. |
|
Aroma threshold values |
Medium strength odor, balsamic type; recommend smelling in a 1.0% solution or less. |
|
General Description |
Lycopene is a naturally occurring red pigment, which belongs to the family of carotenoids. It is found in tomatoes, watermelon and papaya. Lycopene has antioxidant property. |
|
Who Evaluation |
Evaluation year: 2009 |
InChI:InChI=1/C40H56/c1-33(2)19-13-23-37(7)27-17-31-39(9)29-15-25-35(5)21-11-12-22-36(6)26-16-30-40(10)32-18-28-38(8)24-14-20-34(3)4/h11-12,15-22,25-32H,13-14,23-24H2,1-10H3/b12-11+,25-15+,26-16+,31-17+,32-18+,35-21-,36-22+,37-27-,38-28-,39-29-,40-30+
-
A novel route for the total synthesis of...
A semi-preparative HPLC method was devel...
The invention discloses a method for pre...
The present invention relates to methods...
The invention provides a method for prep...
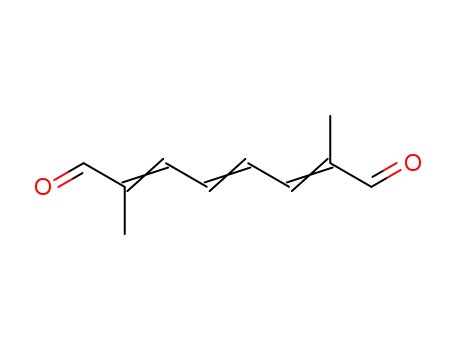
2,7-dimethyl-2,4,6-octanetriene-1,8-dialdehyde

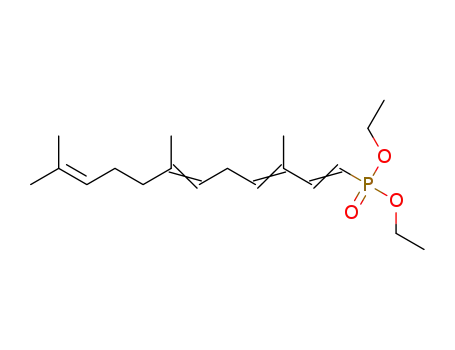
3,7,11-trimethyl-1,3,6,10-tetraene-dodecyl diethyl phosphonate

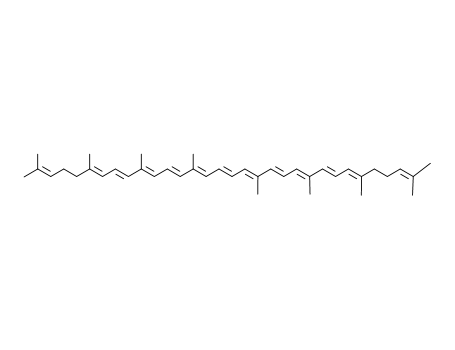
lycopene
| Conditions | Yield |
|---|---|
|
3,7,11-trimethyl-1,3,6,10-tetraene-dodecyl diethyl phosphonate; With potassium tert-butylate; In tetrahydrofuran; dimethyl sulfoxide; at -30 - -25 ℃; for 5h; Inert atmosphere;
2,7-dimethyl-2,4,6-octanetriene-1,8-dialdehyde; In tetrahydrofuran; dimethyl sulfoxide; at -30 - 20 ℃; for 1.25h; Inert atmosphere;
|
59% |
|
3,7,11-trimethyl-1,3,6,10-tetraene-dodecyl diethyl phosphonate; With potassium tert-butylate; In tetrahydrofuran; dimethyl sulfoxide; at 5 ℃; for 2h; Inert atmosphere;
2,7-dimethyl-2,4,6-octanetriene-1,8-dialdehyde; In tetrahydrofuran; dimethyl sulfoxide; at 5 - 25 ℃; for 1.58h;
In chloroform; for 2h; Reflux; Inert atmosphere;
|
52.3% |
|
3,7,11-trimethyl-1,3,6,10-tetraene-dodecyl diethyl phosphonate; With potassium tert-butylate; In tetrahydrofuran; dimethyl sulfoxide; at 5 ℃; for 2h; Inert atmosphere;
2,7-dimethyl-2,4,6-octanetriene-1,8-dialdehyde; In tetrahydrofuran; dimethyl sulfoxide; at 5 - 25 ℃; for 1.58333h;
|
52.3% |
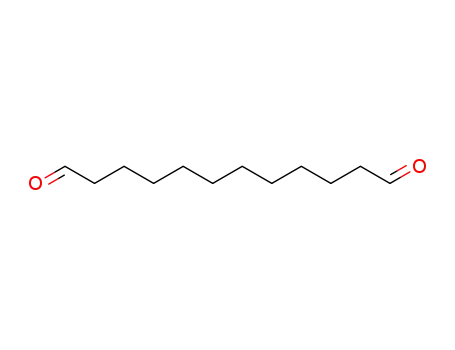
dodecanedial


3,7,11-trimethyl-1,3,6,10-tetraene-dodecyl diethyl phosphonate


lycopene
| Conditions | Yield |
|---|---|
|
3,7,11-trimethyl-1,3,6,10-tetraene-dodecyl diethyl phosphonate; With n-butyllithium; In tetrahydrofuran; hexane; at -40 ℃; for 2h; Inert atmosphere;
dodecanedial; In tetrahydrofuran; hexane; at -40 - 30 ℃; for 1.58333h;
|
71% |
tetrachloromethane
N-Bromosuccinimide
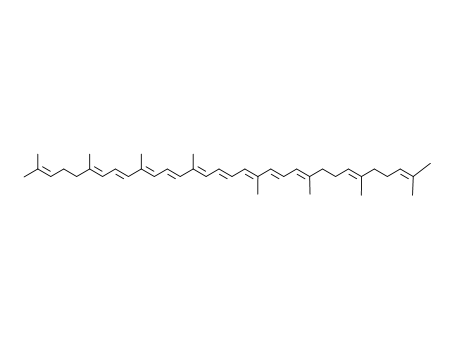
all-trans-neurosporene
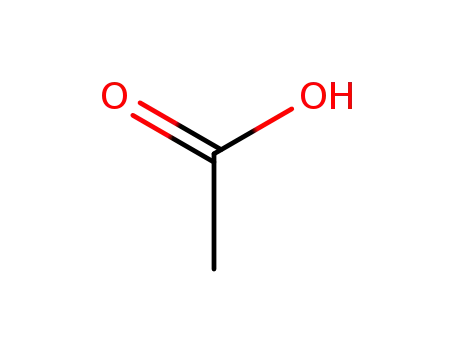
acetic acid
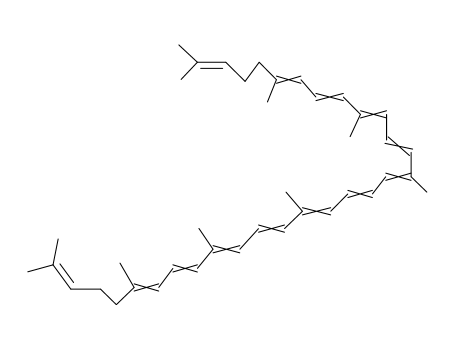
lycopene
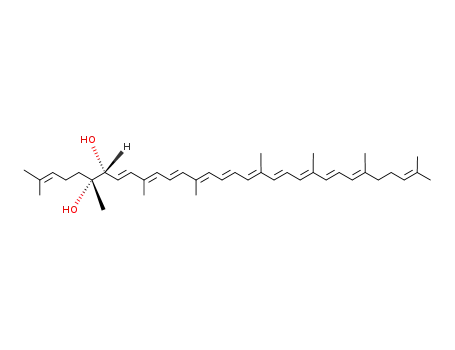
(+/-)-5,6-dihydro-ψ,ψ-carotene-5rF,6tF-diol
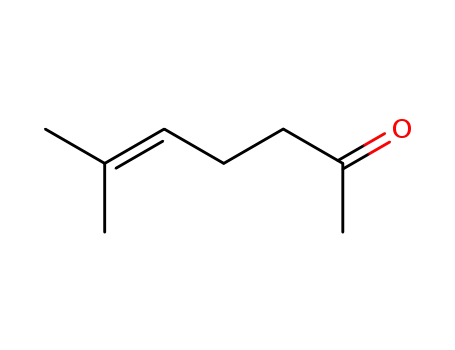
6-Methyl-hept-5-en-2-on
apo-6'-lycopenal